Mon., Feb. 26, 2007
The Experiment #2 reports and the revised
Expt. #1 reports were collected today. It usually takes about a
week to grade new experiment reports. You should expect to see
your Experiment #2 report next Monday or Wednesday. The revised
reports go to the bottom of the grading priority list, you might not
get your report back until after Spring Break.
Optional Assignment #3 is due this
coming Wednesday at the beginning of class. Wednesday is also the
first 1S1P Assignment #2 due date (if you
plan on turning in two reports you must turn in at least one report in
on Wednesday). Copies of the optional assignment and the 1S1P
Assignment #2 worksheets are available in my office (PAS 588).
Here is
what radiative equilibrium (on the earth without an atmosphere) looked
like last Friday. This is a view from outer space.
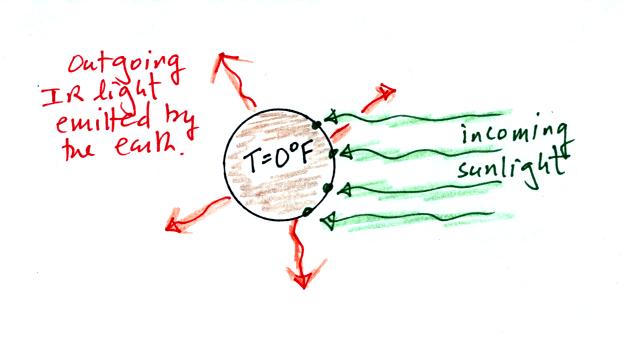
4 units (4 arrows) of incoming sunlight energy is balanced by an equal
amount (4 units = 4 arrows) of outgoing infrared light. Equal
amounts (it doesn't matter if it is different kinds of energy) of
energy be absorbed and emitted.
The next picture shows radiative equilibrium as seen from a
vantage point on the earth's surface.
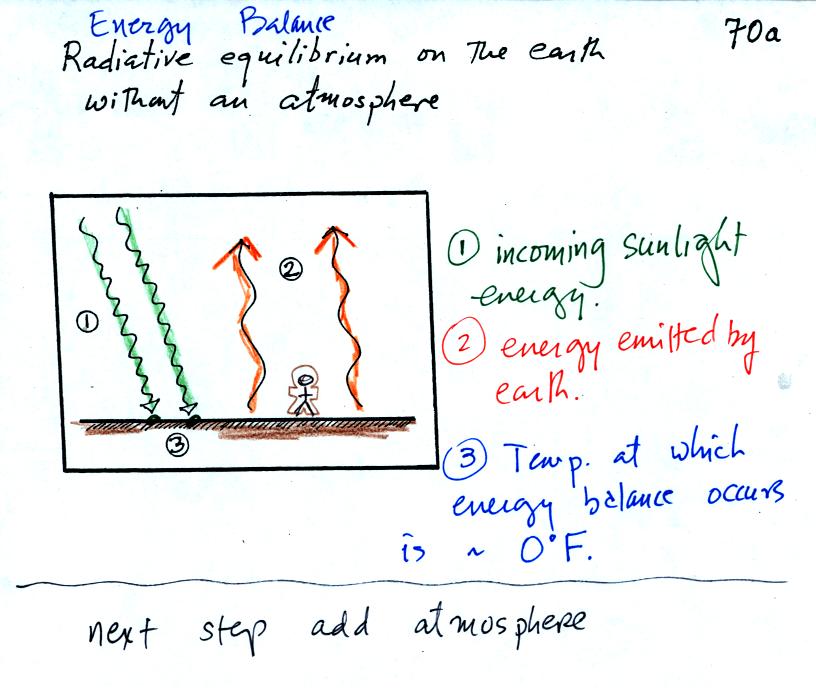
2 arrows of incoming sunlight is balanced by 2 arrows of outgoing IR
radiation.
Before we can add the atmosphere and see how that changes the energy
balance, we need to know something about how the atmosphere affects
different kinds of EM radiation passing through it.

This is a slightly simplified representation of the
filtering effect of
the atmosphere on UV, VIS, and IR light (found on p. 69 in the
photocopied notes, a more realistic version is reproduced on p.
70 in the photocopied class notes). 0% absorption means the
atmosphere behaves like a window
made of clear glass, the air is transparent to light. The light
can
pass freely through the atmosphere. 100% absorption on the other
hand
means the atmosphere is opaque to light, it blocks the light by
absorbing it.
In our simplified representation oxygen and ozone make the atmosphere a
pretty good absorber of UV light The atmosphere is pretty nearly
perfectly
transparent to VIS light (we can check this out with our eyes, we can
see through the air, it is clear). Greenhouse gases make the
atmosphere a
selective absorber of IR light - it absorbs certain IR wavelengths and
transmits others.. Note "the atmospheric window"
centered at 10 micrometers. Light emitted by the earth at this
wavelength will pass through the atmosphere. IR light emitted by
the earth at slightly different wavelengths will be absorbed by
greenhouse gases. It is this ability of H20, CO2,
etc to
selectively absorb certain wavelengths of IR light that is responsible
for the greenhouse effect.
Now the
figure we have all been waiting for, a simplified version of energy
balance on the earth with an atmosphere that does contain green house
gases. Click here if you
want to see the actual figure created in class. Here's a step by
step discussion (perhaps a clearer discussion) of what is shown on that
figure:
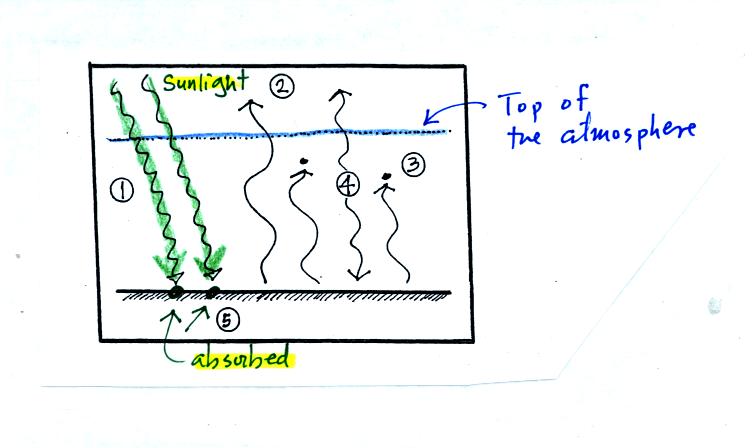
This first picture shows the incoming sunlight (2 units = 2
arrows). We assume that all of it, 100%, is transmitted through
the atmosphere and arrives at the ground and is absorbed.
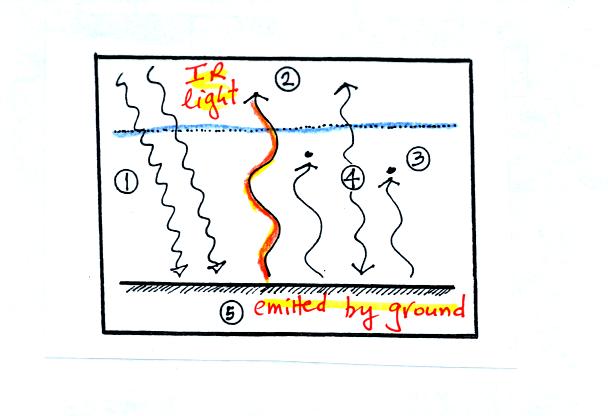
This figure shows IR light being emitted by the ground. It has a
wavelength (near 10 micrometers = the atmospheric window) that is
transmitted through the atmosphere (remember the atmosphere absorbs
some kinds of IR light and transmits others). This light passes
through the atmosphere and goes out into space.
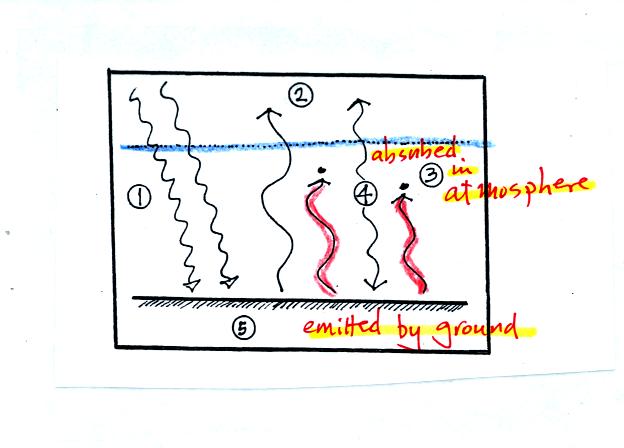
Here the ground is emitting 2 arrows of IR light at a different
wavelength. This radiation is absorbed by gases in the atmosphere.
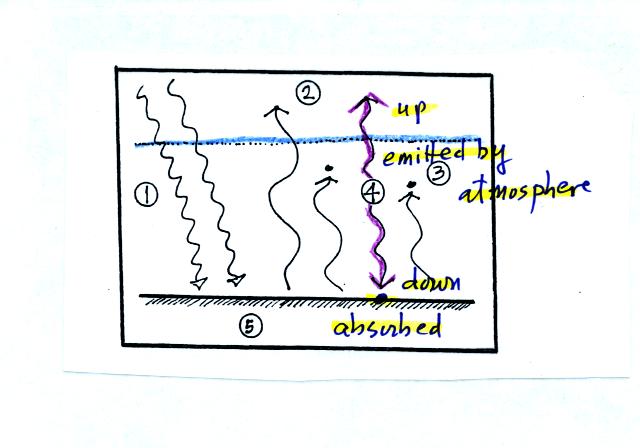
If the atmosphere absorbs 2 arrows worth of energy it must also emit 2
arrows to be in energy balance. It sends one arrow upward into
space. The second arrow is sent down to the ground where it gets
absorbed.
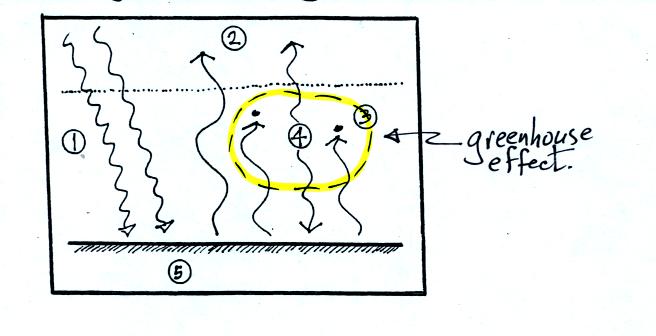
The circled region in the figure is the greenhouse effect believe
it or not (at least a simplified version). The greenhouse effect
is the ability of gases like water vapor and carbon dioxide to absorb
some of the IR light emitted by the ground. These gases in turn
emit IR radiation. Some of this is sent back to the ground.
The ground effectively gets back some of the energy that would
otherwise be lost.
We learned that the surface of the earth is warmer with a greenhouse
effect that it would be otherwise (a global annual average surface
temperature of 60 F rather than 0 F).
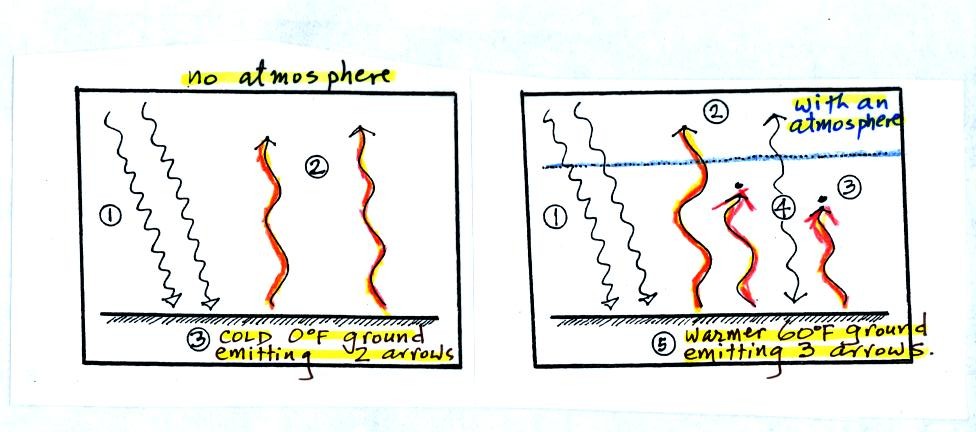
This figure compares energy balance on the earth without an atmosphere
and with an atmosphere containing greenhouse gases (the example we just
finished analyzing).
At left energy balance is achieved when the ground is 0 F and is
emitting 2 arrows of energy, the same amount of energy it is getting
from the sun.
At right the ground emits 3 arrows of energy. The Stefan
Boltzmann law tells us that the amount of energy emitted by an object
depends on temperature (to the 4th power). The ground must be
warmer (warmer than 0 F) in the figure at right in order to be emitting
3 arrows of energy. The ground at right is a pleasant 60 F.
The next figure (not shown or
discussed in class) shows another way of trying to understand
why or how the greenhouse effect makes the earth's surface warmer.
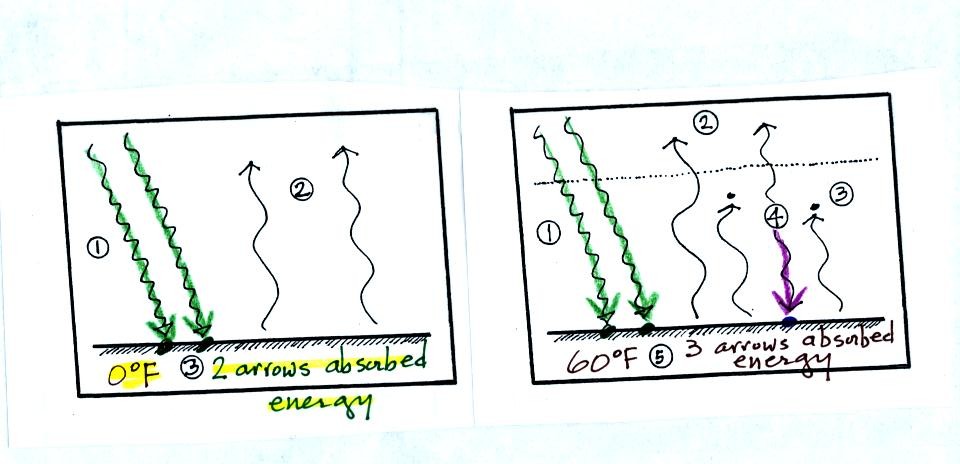
At left the ground absorbs 2 arrows of energy and warms to 0 F.
The ground at right is absorbing 3 arrows of energy (2 from the sun and
1 coming from the atmosphere). Doesn't it make sense that the
ground at right will be warmer than the ground at left?
We've just
analyzed a simplified version of energy balance on the earth.
Here's a more realistic look at energy balance.

In our simplified version of the greenhouse effect we
assumed that
100%
of the sunlight arriving at the top of the atmosphere passes through
the atmosphere and gets absorbed by the ground. The bottom figure
above shows that in reality only about 50% of the incoming sunlight
gets absorbed at the ground.
About 20% of the incoming sunlight is absorbed by gases in the
atmosphere. Sunlight is a
mixture of UV, VIS, and IR.
Ozone and oxygen will absorb a lot of the UV (though there isn't much
UV in sunlight) and greenhouse gases will absorb some of the IR
radiation in sunlight (IR light accounts for about half of the light in
sunlight).
The figure above shows what happens to the incoming sunlight. The
figure on p. 72 in the photocopied class notes adds the energy emitted
by the ground together with energy absorbed and emitted by the
atmosphere. Click here
if you want to see the figure created in
class.
Below we'll break the figure down into manageable
pieces. We will
start with the emission of energy by the atmosphere.
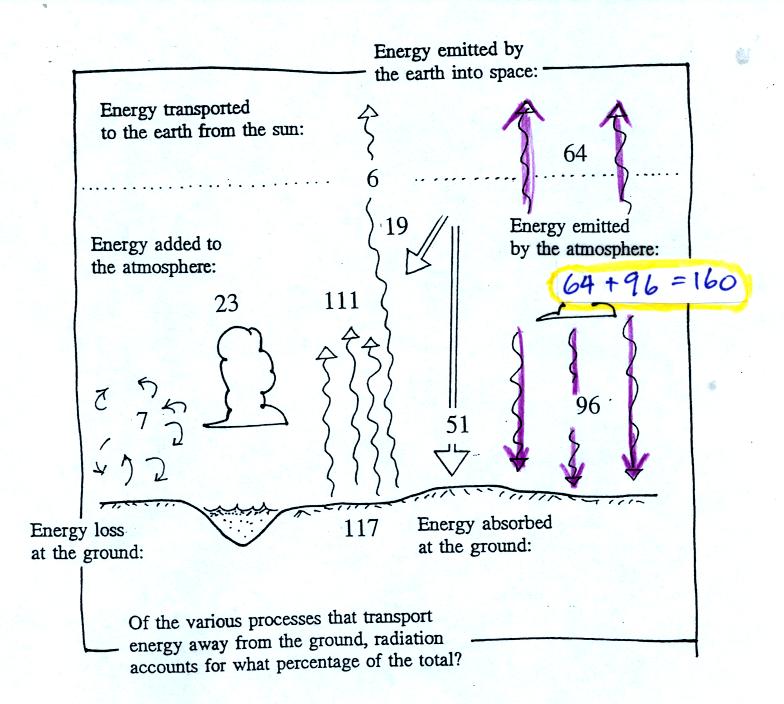
The atmosphere emits a total of 160 units of energy: 64 units
upward into space and 96 units downward
to the ground. Why the difference? That is a what
determines how much energy an object can emit question. The
answer is temperature. Warm objects emit
more energy (or emit energy at a higher rate) that cold objects.
The upper atmosphere must be colder than the lower atmosphere.
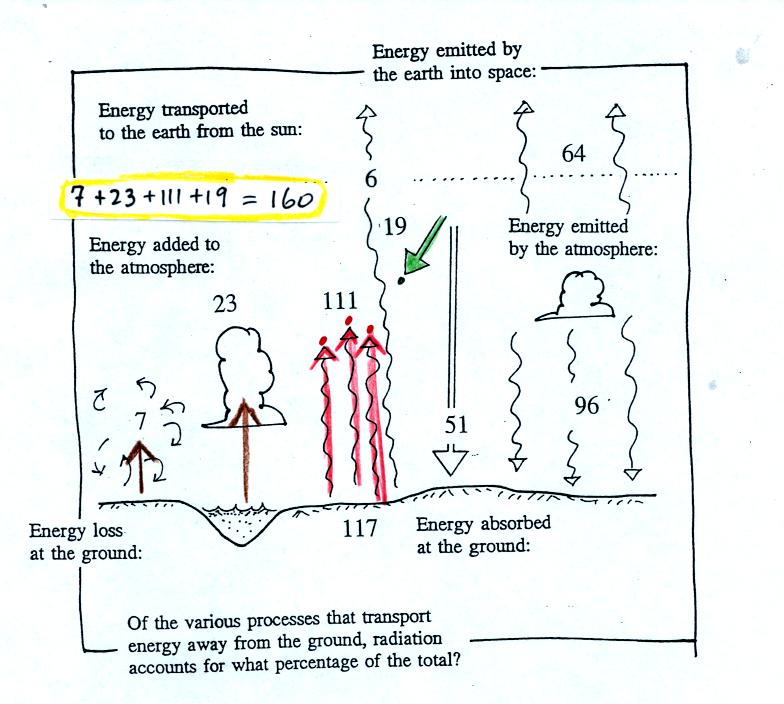
This figure shows the energy being added to the
atmosphere. 19
units of sunlight are absorbed, 111 units of IR radiation emitted by
the earth are absorbed. This gives 130 units, we need a total of
160. Conduction and convection transport only 7 units, latent
heat transports 23 units from the ground to the atmosphere. They
account for the missing 30 units.
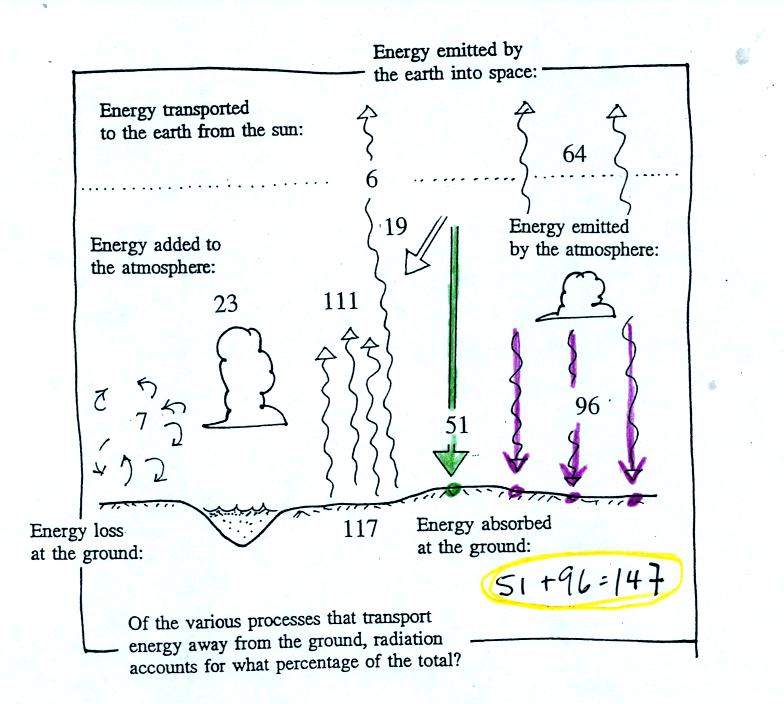
The ground absorbs 51 units of sunlight and 96 units of
energy emitted
by the atmosphere. On average the ground gets more energy from
the atmosphere than it does from the sun.
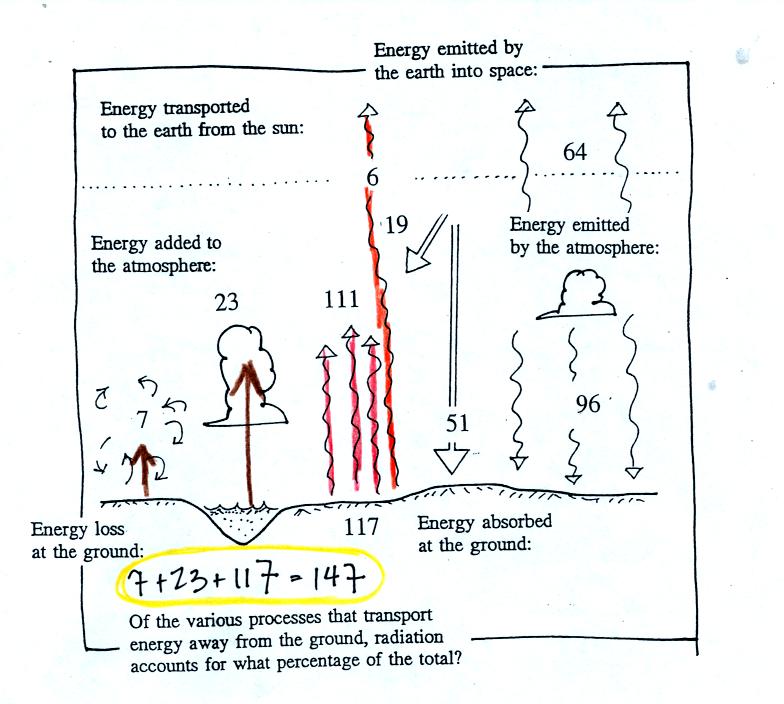
The ground loses 117 units of energy by emitting IR
radiation.
Conduction, convection, and latent heat transport an additional 30
units away from the ground. That gives a total of 147 units, so
the ground is in energy balance. Note that 117 out of the total
147 units, 80%, lost by the ground is in the form ofEM radiation.

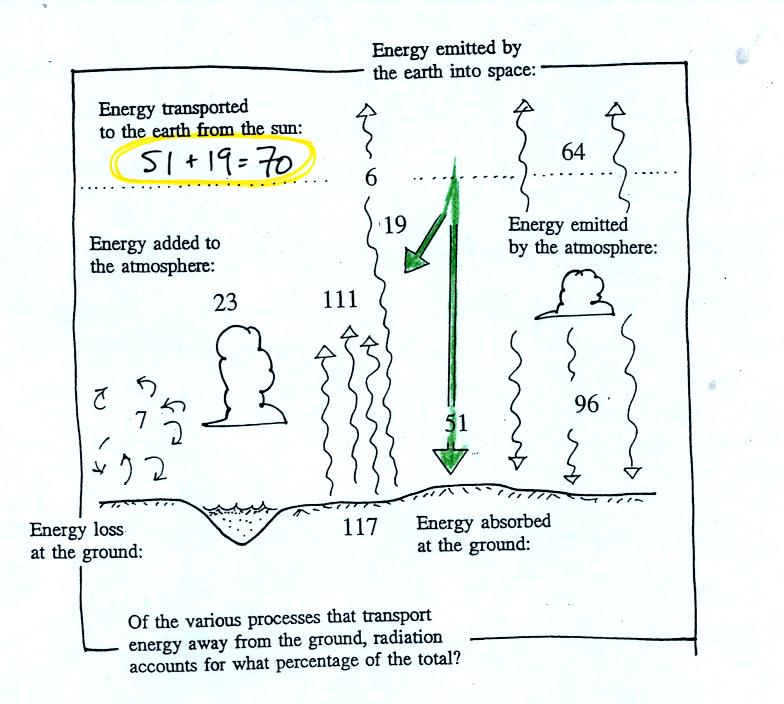
The earth and atmosphere together send 70 units into space
which balances the 70
units of sunlight absorbed by the atmosphere or the ground shown below.
There was
an optional in class assignment question given in class. Here it
is:
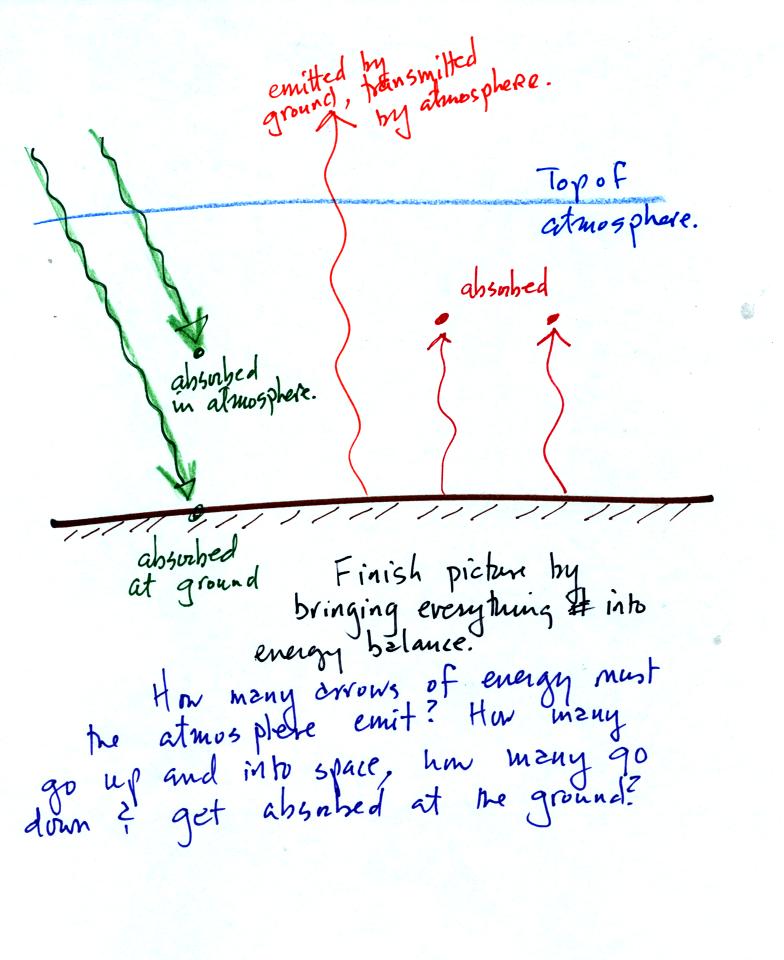
2 arrows of sunlight energy arrive at the earth. 1 arrow is
absorbed by the atmosphere, the other passes through the atmosphere and
gets absorbed by the ground. The ground emits 3 arrows of IR
light, 1 passes through the atmosphere and goes into space. The
other two are absorbed by the atmosphere.
Your job is to bring the picture into energy balance. How many
arrows of energy must the atmosphere emit. How many should be
directed upward and go into space, how many should go downward and be
absorbed at the ground.
Put your answer on a sheet of paper with your name and turn it in at
the beginning of class on Wednesday if you want a little extra credit.

















