Fri., Jan. 19, 2007
We began class with a quick look at the forecast
for this weekend provided by the Tucson
National Weather Service website. You can access NWS web
pages for other locations in the US by going to www.weather.gov.
There is a chance of rain beginning to night and extending through the
beginning of the weekend. We also looked at
infrared satellite photographs of moisture and clouds streaming
into Arizona from the SW and a low pressure center located off the
California coast.
We'll be
jumping back between stratospheric and tropospheric ozone today.

We'll eventually be making some photochemical smog as a class
demonstration. This will require ozone (and a hydrocarbon of some
kind). We'll use the simple stratospheric process for making
ozone in the demonstration rather than the more complex tropospheric
process.
In the stratosphere ozone is beneficial because it absorbs dangerous
high-energy ultraviolet light.

Some of the serious hazards posed by ultraviolet light that
manages to reach the ground are listed above (on p. 18 in the
photocopied class notes).

Human activities add substances to the atmosphere that can
potentially
reduce ozone concentration in the ozone layer (which would result in
increased exposure to UV light at the ground).
Chlorofluorocarbons now probably represent the greatest threat to the
ozone layer. The next figure shows how CFC compounds react with
and destroy stratospheric ozone.
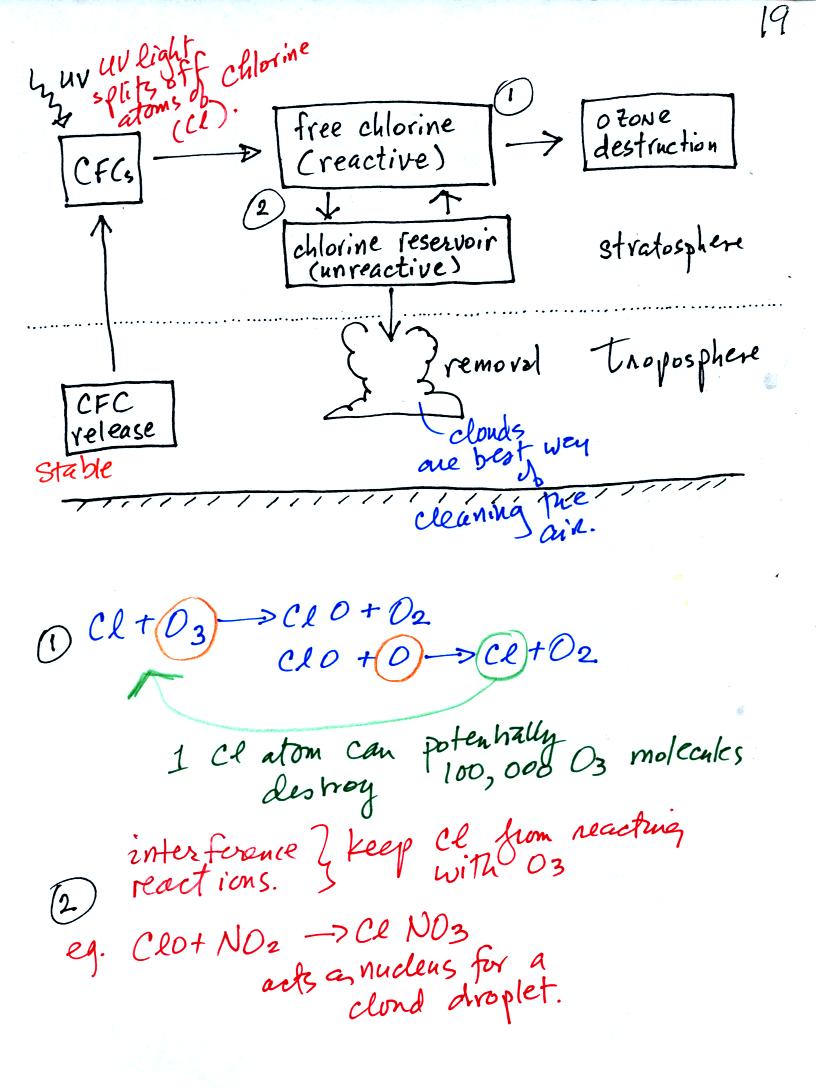
Because CFC molecules are normally very stable (it is hard
to break the
molecule apart) and unreactive, they can remain in the atmosphere a
long
time. This gives them time to move into the stratosphere.
Once there UV light can break atoms of chlorine (Cl) off the CFC
molecules. This free chlorine can react with and destroy ozone
(shown in Equation 1 above). Note how chlorine reappears at the
end of the
two step reaction and can destroy additional ozone.
Sometimes the chlorine will react with other substances and will be
removed from the atmosphere (Equation 2 above).
Now back
to tropospheric ozone and photochemical smog.
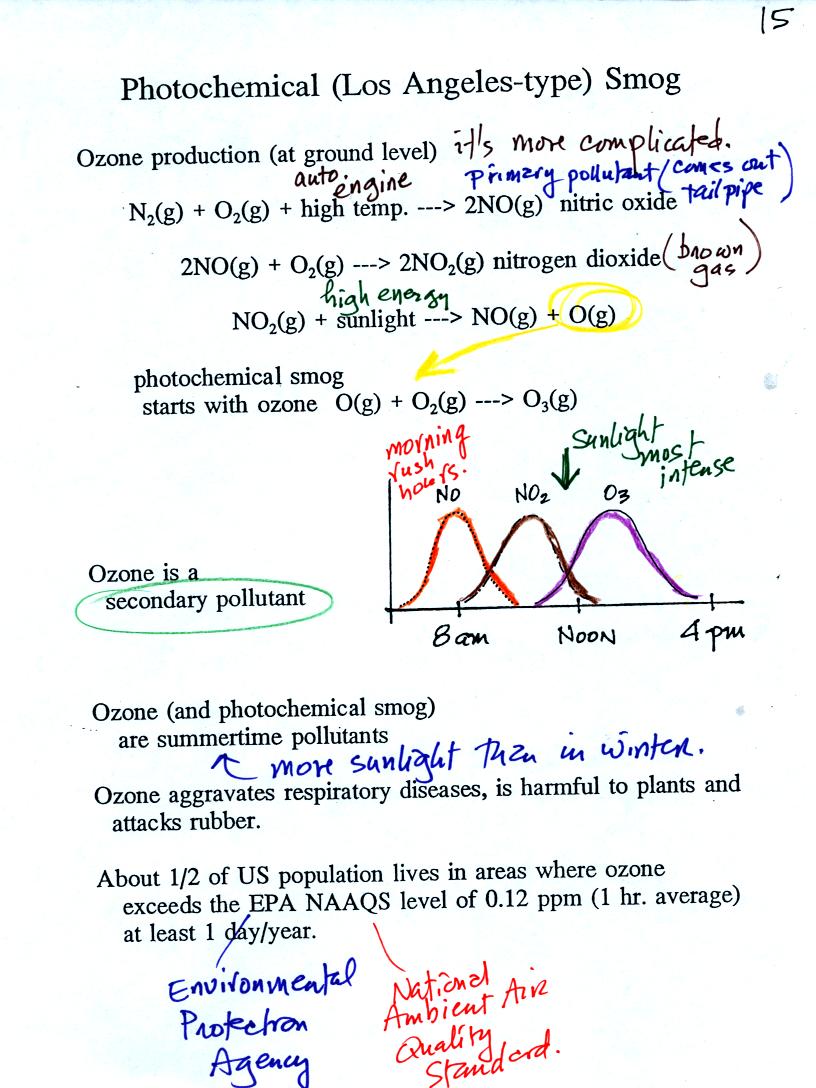
At the top of this figure you see that a more complex series of
reactions is responsible for the production of tropospheric
ozone. The production of tropospheric
ozone begins with nitric
oxide
(NO). NO is produced when nitrogen and oxygen are heated (in an
automobile engine for exampe) and react. The NO can then react
with oxygen to make nitrogen dioxide, a poisonous brown-colored
gas. Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2
react (just
as they do in the stratosphere) to make ozone (O3).
Because ozone
does not come directly from an automobile tailpipe or factory chimney,
but only shows up after a series of reactions, it is a secondary
pollutant. Nitric oxide would be the primary pollutant in
this example.
NO is produced early in the day (during the morning rush hour).
The concentration of NO2
peaks
somewhat later. Peak ozone concentrations are usually found in
the afternoon. Ozone concentrations are also usually higher in
the summer than in the winter. This is because sunlight plays a
role in ozone production and summer sunlight is more intense than
winter sunlight.
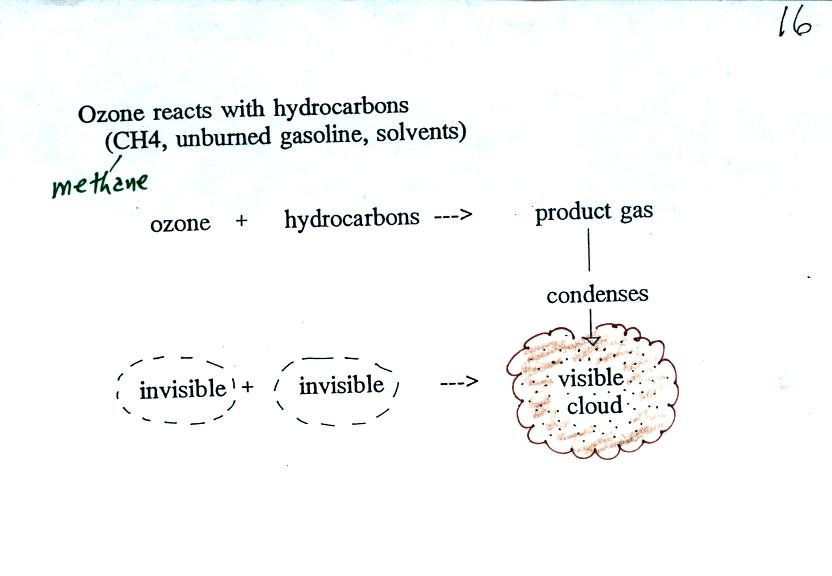
As shown in the figure below,
invisible ozone can react with a hydrocarbon of some kind which is also
invisible to make a
product
gas. This product gas sometimes condenses to make a visible smog
cloud or haze.
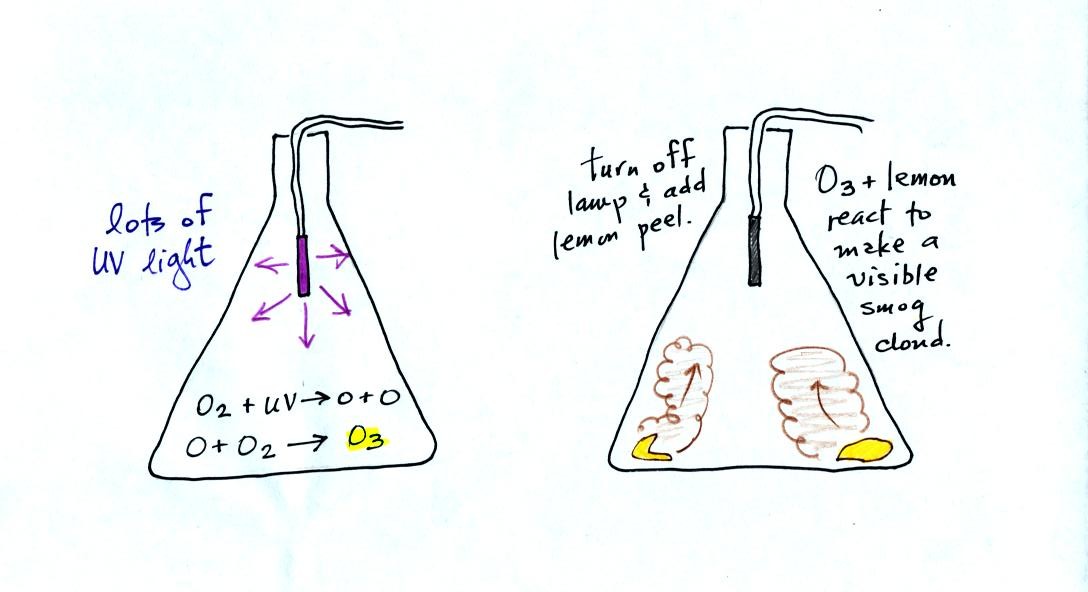
The class demonstration of photochemical smog is summarized
below (a flash was used instead of the aquarium shown on the bottom of
p. 16 in the photocopied class notes). We begin by using the UV
lamp to fill the flask with
ozone. Then a few pieces of fresh lemon peel were added to the
flask. A whitish cloud quickly became visible (colored brown in
the figure below).
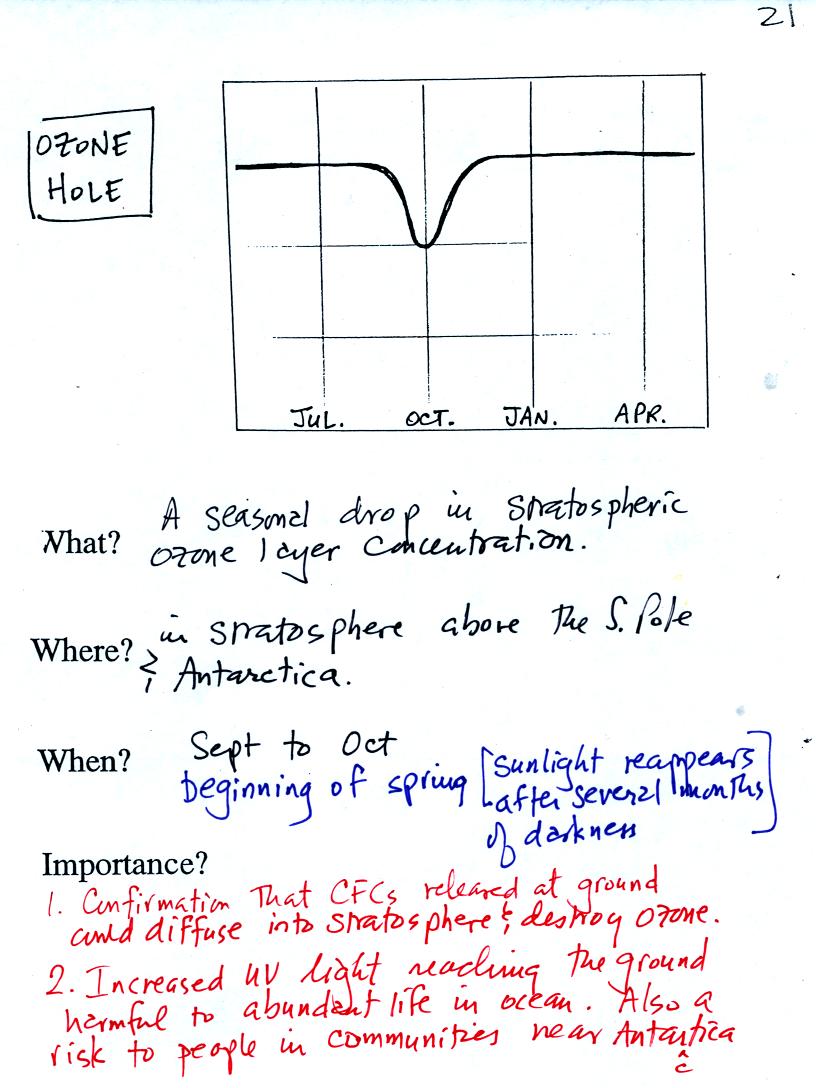
The ozone hole that forms above the S. Pole every year around October
was one of the first real indications that CFCs could react with
and destroy stratospheric ozone. Note that the ozone in the ozone
layer doesn't disappear completely, rather the concentration drops
significantly. The ozone hole is only present for a couple of
months every year.

The discussion above explains how extremely cold
temperatures and an
unusual wind pattern above the S. Pole in the winter are thought to
create the ozone hole when the sun returns in the spring.
Basically a new series of reactions (that take place on the surface of
cloud particles) interfere with the interference reactions. The
interference reactions would keep chlorine from reacting with and
destroying ozone. Interfering with those interference reactions
again makes chlorine available to react with and destroy ozone.
Chlorine containing compounds build up during the winter and are able
to destroy ozone once the sun returns in the spring.
One last but important point that
I forgot to mention in class.

Many people think that thinning of the ozone layer and increased
amounts of UV
light reaching the ground is the cause of global warming.
This is NOT correct. Increasing concentrations of greenhouse
gases like carbon dioxide and
enhancement of the greenhouse effect are the cause of global warming.









