Wednesday Jan. 31, 2007
A new optional assignment was handed
out in class today. It is due one week from today on Wed., Feb. 7.
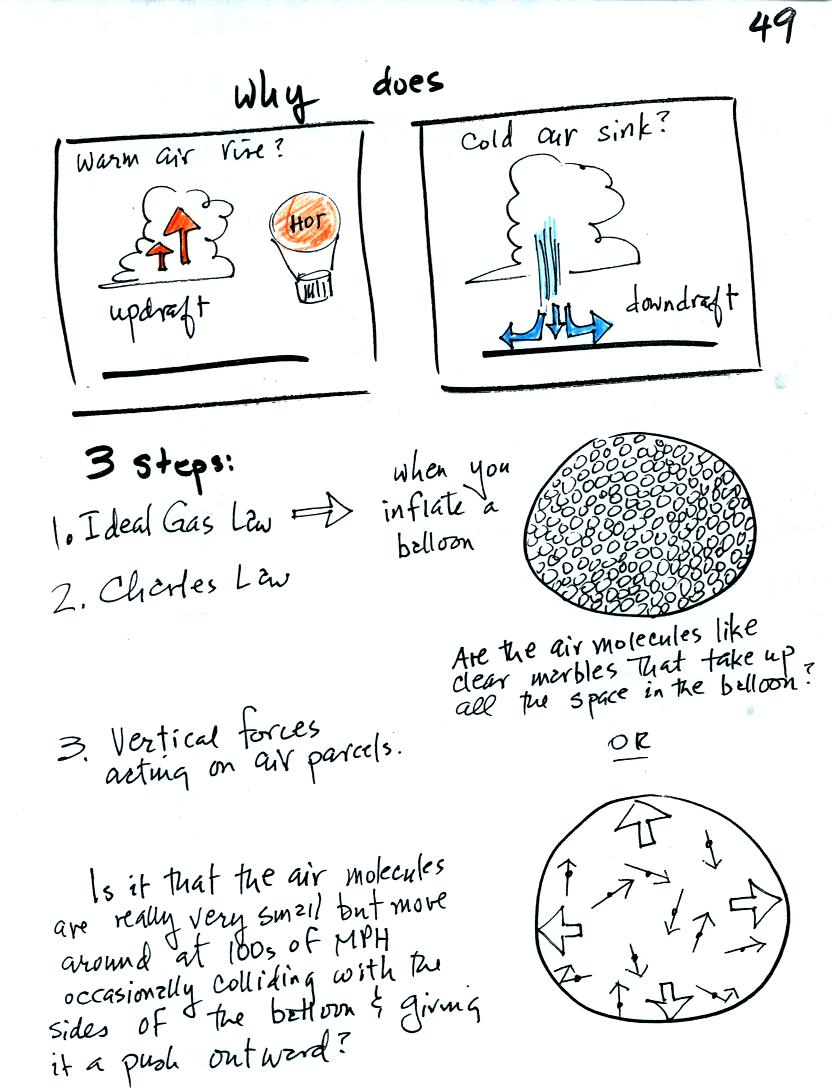
We will learn a little bit about the ideal gas law today before
the Practice Quiz. This is the first of three steps that will
lead us to an understanding of why warm air rises and cold air sinks.

If you could look inside a balloon you
wouldn't see the inside of the balloon literally filled with air
molecules as depicted above at right.
You would see that there was an enormously large number of gas
molecules moving in random directions at 100s of MPH but you would find
that most of the volume inside the balloon was empty space. Air
molecules colliding with the inside walls of the balloon push outward
and keep the balloon inflated.
The ideal gas law equations tell us how variables like
the number of gas
molecules,
the volume of the balloon, and
the density and temperature of the air
affect the pressure of the air in the
balloon.
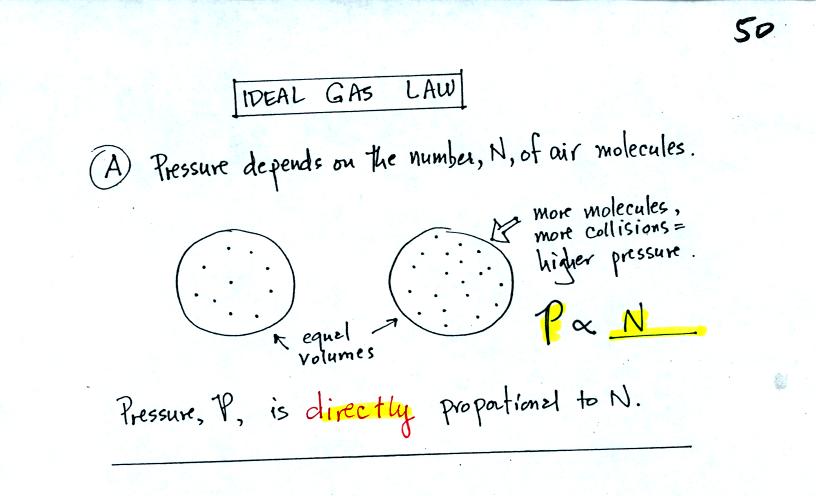
The pressure produced by the air
molecules inside a balloon will
first depend on how many air molecules are there.
If there weren't any air molecules at all there wouldn't be any
pressure. As you add more and more add to something like a
bicycle tire, the
pressure increases. Pressure is directly proportional to N (an
increase in N causes an increase in P).
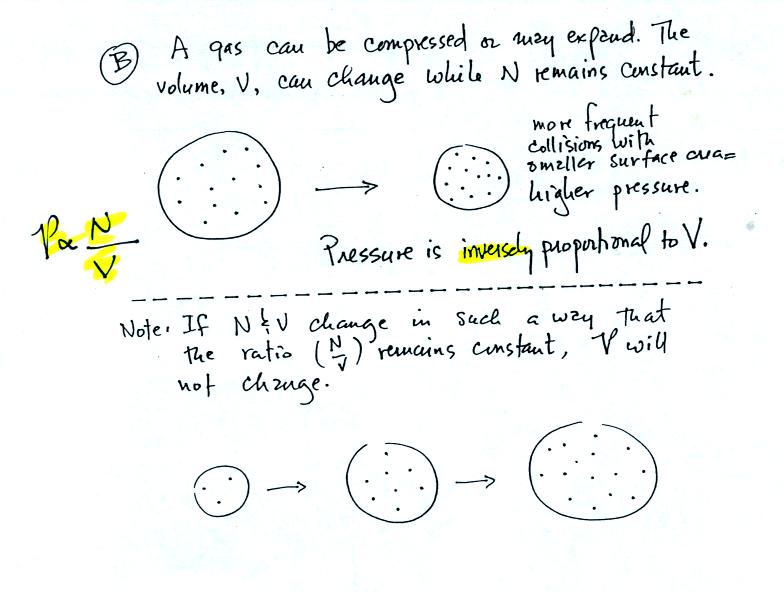
Air pressure inside a balloon also
depends on the size of the
balloon. Pressure is inversely proportional to volume, V
(increasing V decreases P and vice versa).
Note it is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
Experiment #1 that some of you are working on. Oxygen in a
graduated cylinder reacts with steel wool to form rust. Oxygen is
removed from the air sample which is a decrease in N. As oxygen
is removed, water rises up into the cylinder decreasing the air sample
volume. N and V both decrease in the same relative amounts and
the air sample pressure remains constant.
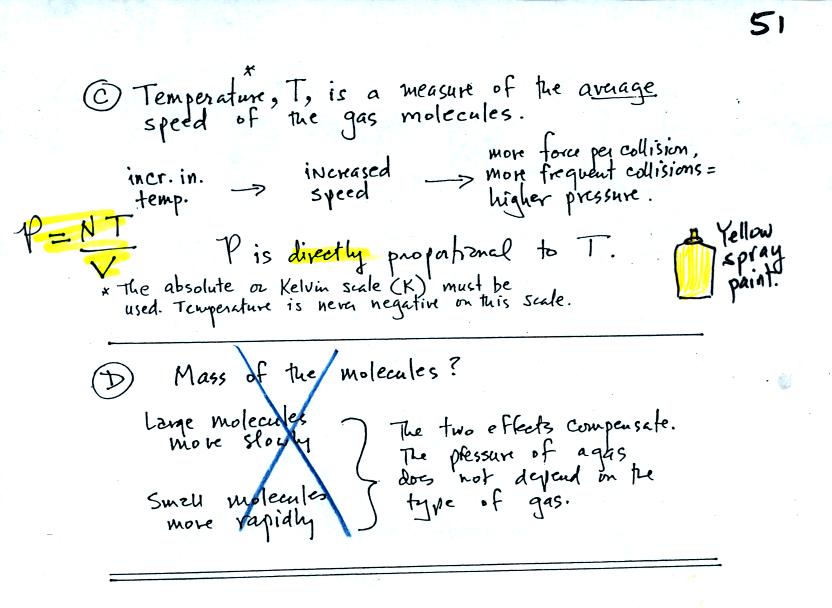
Increasing
the temperature of the gas in a balloon will cause the gas molecules to
move more quickly. They'll collide with the walls of the balloon
more frequently and rebound with greater force. Both will
increase the pressure.
You
shouldn't throw a can of spray paint into a fire. The
pressure of the gas inside a container depends on the gas
temperature. If the can gets hot enough, the buildup in pressure
could cause the can to rupture.
Surprisingly the pressure does
not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
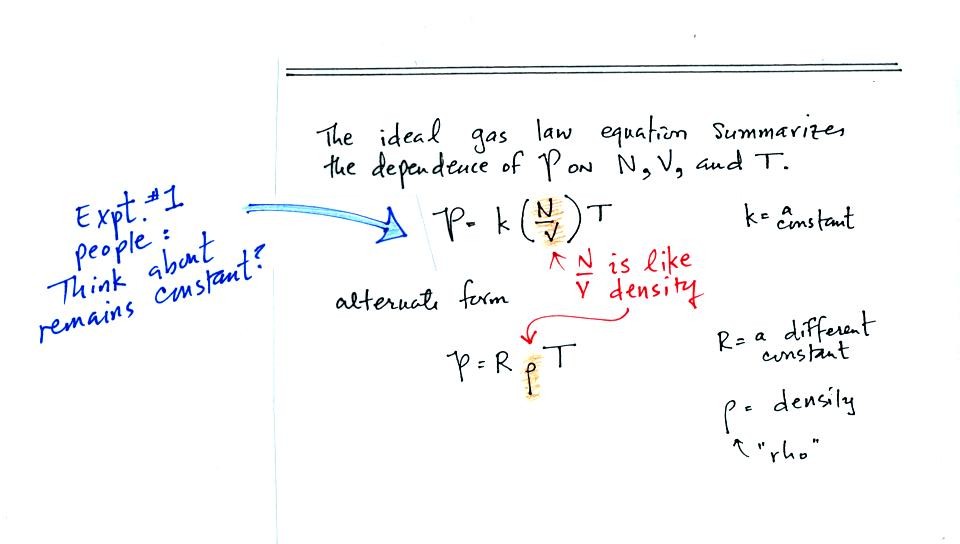
Here are the two ideal gas law equations. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers.
(1) Pressure = (Number of air molecules) multiplied by temperature divided by volume
or
(2) Pressure = (density) multiplied
by (temperature)
The Expt. #1 people will use Eqn. (1) in their reports. They
should be thinking about what variables in the equation remain constant
and which ones change.
Here is the ideal
gas law animation shown in class. You
can vary N, V, or T and see the effect on pressure.





