
The fact that the two objects are balanced in Question #2 tells
you that they both have the same weight. In order to have the
same weights they must have the same mass.
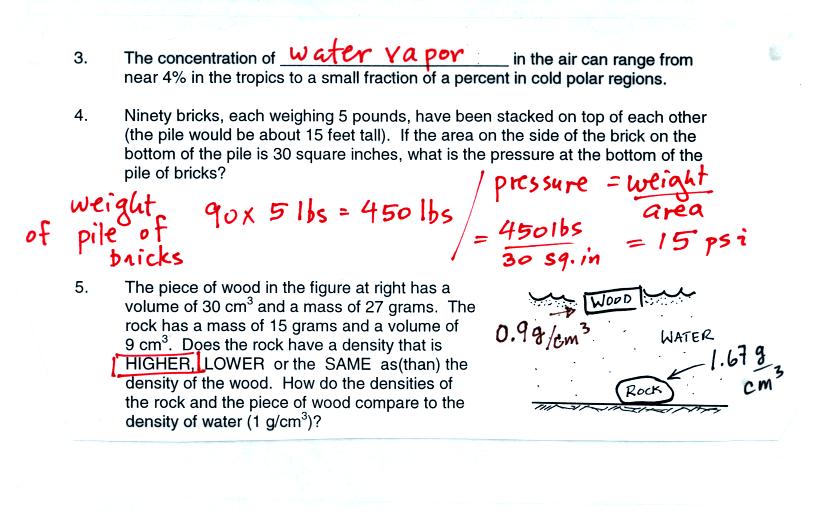
The wood is floating. It must have a density less than
water. Density is mass/volume. You can compute the density
of the wood by dividing its mass, 27 g, by its volume, 30 cm3.
The density is 0.9 g/cm3. The density of the rock is greater than
water. The density of the rock is 12 g/ 9 cm3 or 1.67
g/cm3.

Typical sea level pressure is 14.7 pounds per square inch which is
about 1000 mb, 30 inches of mercury, or 1 bar. 1 bar is therefore
about 15 psi.

Pressure at any level in the atmosphere is determined by the weight of
the air overhead. As you move upward in the atmosphere there is
less and less air above, less and less weight, pressure decreases with
increasing altitude. Pressure decreases most rapidly with
increasing altitude in dense air. The densest air in the
atmosphere is found at ground level.

The red shaded portion in the barometer (between A and C) acts like a
balance. The pressure produced by the weight of a column of air
above and pushing down on Point C is balanced by the weight and
pressure of the mercury column extending from C to D (shaded
orange). The pressures at Points A anc C are equal. We
assume there is no pressure pushing down on the top of the mercury
column at Point D.

Pressure decreases with increasing altitude. The pressure at
Point A will be a little higher than the pressures at the middle and
top of the balloon. The numbers (15, 14.5, and 14) were added to
illustrate the decrease of pressure with increasing altitude.

A balloon will expand or shrink in order to keep the pressure of the
air inside the balloon equal to the pressure of the air outside the
balloon. Heating the air in a balloon would momentarily increase
the pressure of the air in the balloon and the balloon would expand (we
will assume that this happens so quickly that pressure essentially
remains constant). Air hasn't been added to or removed from the
balloon. As the balloon expands and its volume increases, the
density will decrease. Air pressure remains constant.
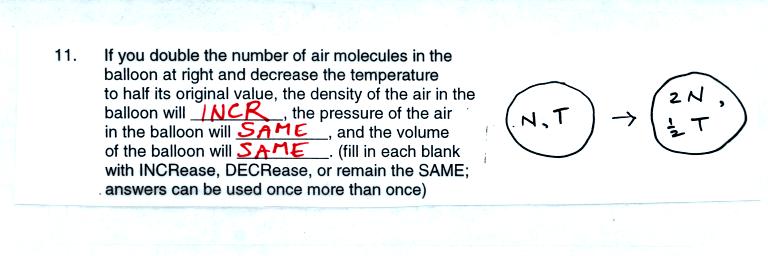
Ordinarily adding air to a balloon would momentarily increase the
pressure. But we cool the air at the same time. Cooling the
air would momentarily decrease the pressure. So it would be
possible to add air and cool the air at the same time and keep the
pressure constant. If the pressure remains constant there is no
reason for the balloon to expand or shrink, thus the volume remains
constant.

Given time the body will adapt to the high altitude conditions by
increasing the number of red blood cells in the blood. Back down
at sea level your body would then be able to transport more oxygen and
your performance would be enhanced.










