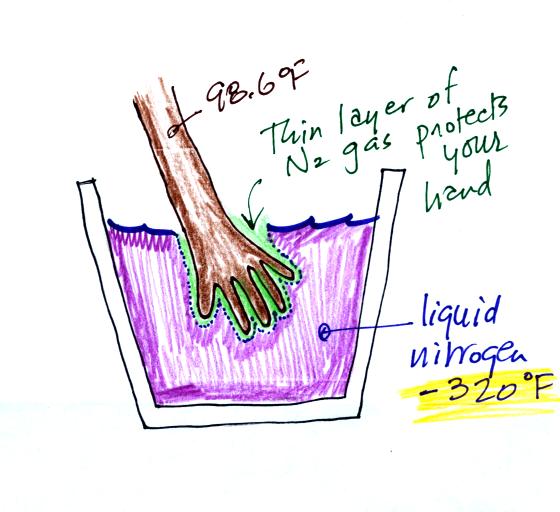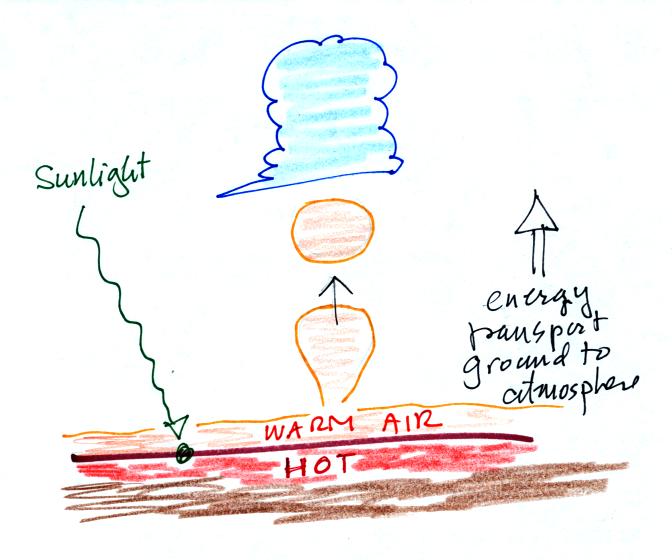
Convection is one of 4
ways causing air to rise. Rising air expands, cools, and can form
clouds; most of the summer thunderstorms in Tucson are convective
storms. Convection is also an energy transport process. The
figure above shows sunlight arriving at the ground, being absorbed, and
heating the ground. The ground in turns warms air in contact with
the ground. Little "parcels" of warm low density rise and carry
heat vertically from the ground to higher in the atmosphere.
Latent
heat energy transport was the final topic of the day.
Energy
transport in the form of latent heat is the second most important
energy transport process (second only to electromagnetic
radiation). A copper bar was heated with a propane torch.
How could you cool the bar? You could just hold onto it and let
conduction and free convection carry away heat. It might take
several minutes for the bar to cool. You cold blow on the bar,
forced convection would cool it a little more quickly. The
fastest way to cool the bar is to plunge it into a glass of
water. The "pssst" that that you heard in class was boiling
(evaporating) water. This was an illustration of latent heat
energy transport and cooled the bar in a few seconds.
Latent heat energy transport is sometimes a little hard to visualize
or understand because the energy is "hidden" in water vapor or water.

Latent heat energy transport is associated with changes of
phase (solid to liquid, water to water vapor, that sort of thing) A
solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously add or supply the energy (such as when you put
water in a
pan and put the pan on a hot stove) or the needed energy will be
taken from the surroundings (from your body when you step out of a
shower in the morning).
A 240 pound man (or woman) running at 20 MPH has just
enough
kinetic energy (if you could somehow capture it) to
be able to melt an ordinary ice cube. It would take 8 people to
evaporate the resulting water.
You can consciously remove energy from water vapor to make
it
condense
or from water to cause it to free (you could put water in a
freezer; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm). Note the orange energy arrows have
turned around and are pointing from the material toward the
surroundings.
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more
rapidly. The condensation may actually be the dominant process.
You feel cold when you step out of a shower and water on
your body
evaporates. The opposite situation, stepping outdoors on a humid
day
and actually having water vapor condense onto your body (it can happen
to your sunglasses but not to you, your body is too warm). If it
did
happen it would warm you up.
The story starts at left in the
tropics where there is often an abundance or surplus of sunlight
energy. Some of the incoming
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.
We'll add an additional detail to an earlier picture.
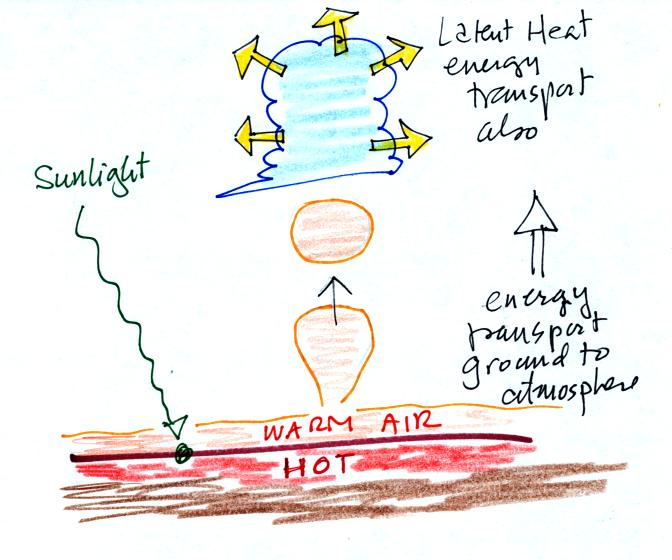
The formation of a cloud means that
latent heat is being released into the air. Two energy transport
processes are at work in this picture: convection and latent heat
(conduction is also present, that is how is energy is transported from
the hot ground into the thin layer of air in contact with the ground.