Monday Sep. 8, 2008
click here for printer friendly version
in Microsoft WORD format
Today's musical selection "Entre de los Aguas" was from a local
group: Tesoro.
Hurricane Ike is going to miss
Florida, it now appears to be headed for the coast of Texas.
Now some good news, a Bonus 1S1P Assignment.
We quickly
reviewed the natural and anthropogenic processes that add carbon
dioxide to the atmosphere and remove CO2 from the atmosphere.
You'll find these at the end of Fri., Sept. 5
class notes.
To
really understand
why human activities are causing atmospheric CO2
concentration to
increase we need to look at the relative amounts of CO2
being added to
and being removed from the atmosphere (like amounts of money moving
into and out of a bank account and their effect on the account
balance). A simplified version of the carbon cycle is shown
below.
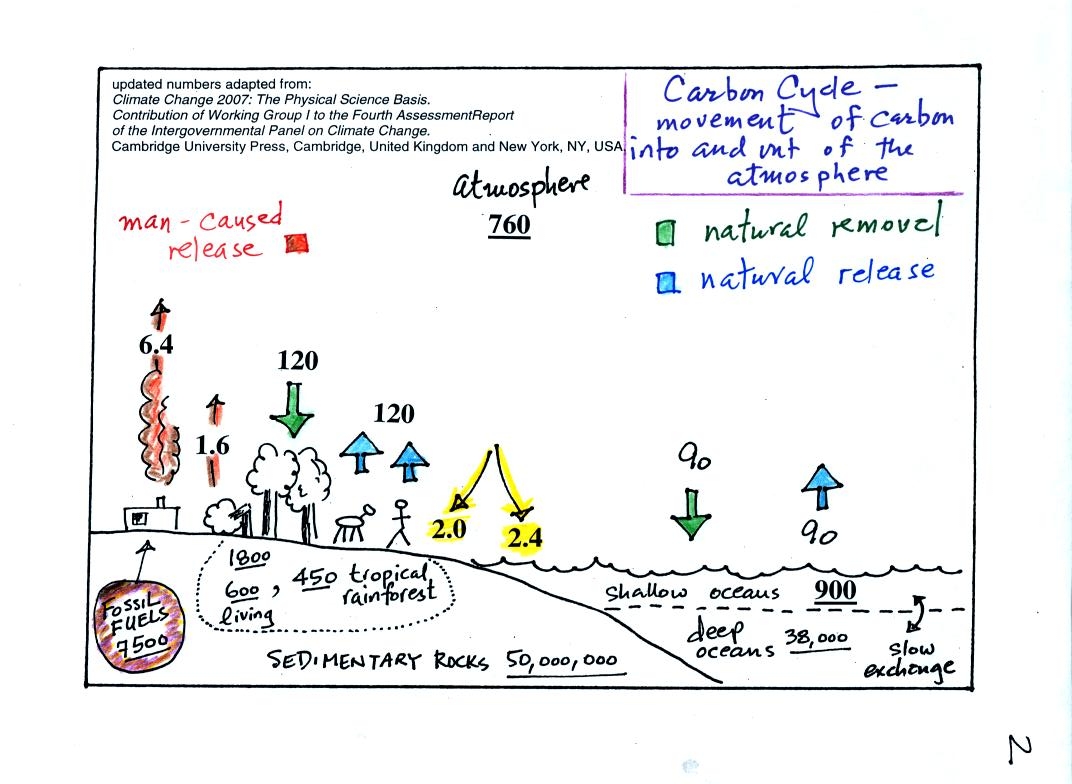
Here are the main points to take from this figure:
1. The underlined numbers show
the amount of carbon stored in "reservoirs." For example 760
units* of carbon
are stored in the atmosphere (predominantly in the form of CO2,
but
also in small amounts of CH4 (methane),
CFCs
and other gases; carbon is found in each of those
molecules). The other numbers show
"fluxes," the amount of carbon moving into or out of the atmosphere
every
year. Over land, respiration and decay add 120 units* of carbon
to the
atmosphere every year. Photosynthesis (primarily) removes 120
units every year.
2. Note the natural processes
are in balance (over land: 120 units added and 120 units removed, over
the oceans: 90 units added balanced by 90 units of carbon removed from
the atmosphere every year). If these were the only processes present,
the atmospheric concentration (760 units)
wouldn't change.
3. Anthropogenic (man caused) emissions
of
carbon into the air are small compared to natural processes. About
6.4 units are added during combustion of fossil fuels and 1.6
units are added every year because of deforestation (when trees are cut
down they decay and add CO2 to the air, also because they
are dead they
aren't able to remove CO2 from the air by photosynthesis)
The rate at which carbon is added to the atmosphere by man is not
balanced by an equal rate of removal: 4.4 of the 8 units added every
year are removed (highlighted in yellow in the figure). This
small imbalance (8 - 4.4 = 3.6 units of carbon are left in the
atmosphere every year) explains why
atmospheric carbon dioxide concentrations are increasing with time.
4. In the next 100 years or so,
the 7500 units of carbon stored in the fossil fuels reservoir (lower
left
hand corner of the figure) will be dug up or pumped out of the ground
and burned. That will add 7500 units of carbon to the air.
The big
question is how will the atmospheric
concentration change and what effects will that have on climate?
*don't worry about the units. But here they are
just in case you are interested: Gtons (reservoirs) or Gtons/year
(fluxes)
Gtons = 1012 metric tons. (1 metric ton is 1000
kilograms or
about 2200
pounds)
So here's where we're at in our discussion
of climate change and global warming:
Atmospheric CO2 concentration was fairly constant between
1000 AD and
the mid
1700s.
CO2 concentration has been increasing since the
mid
1700s (other greenhouse gas concentrations have also been
increasing).
The concern is that this might enhance or strengthen
the
greenhouse effect and cause global warming.
The obvious question is what has
the temperature of the earth been doing during this period? In
particular has there been any warming associated with the increases in
greenhouse gases that have occurred since the mid 1700s?
We must address the temperature question in two parts.
First part:
Actual accurate
measurements of temperature (on land and at sea)
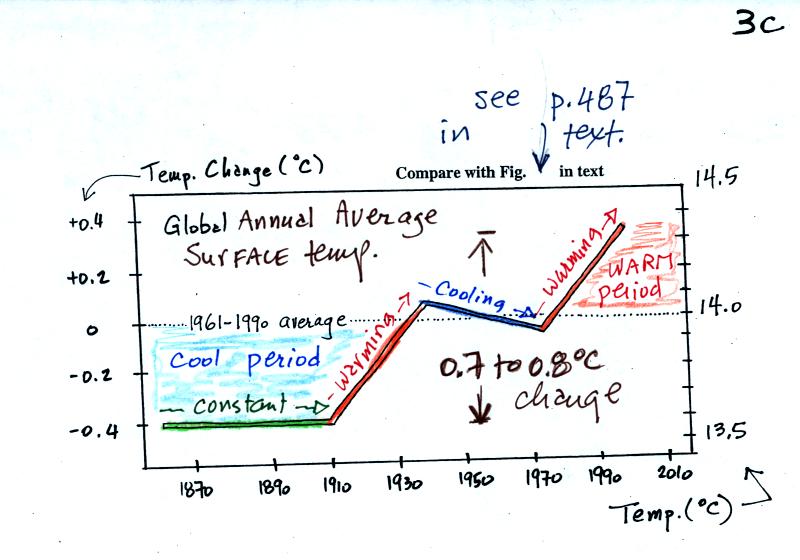
Temperature appears to have
increased 0.7o to 0.8o
C during this
period. The increase hasn't been steady as you might have
expected given
the steady rise in CO2 concentration; temperature even
decreased slightly between about 1940 and 1970.
It is very difficult to detect a temperature change this small over
this period of time. The instruments used to measure temperature
have changed. The locations at which temperature measurements
have been made have also changed (imagine what Tucson was like 130
years ago). About 2/3rds of the earth's surface is ocean.
Sea surface temperatures can now be measured using satellites. Average
surface temperatures naturally change a lot
from year to year.
The year to year variation has been left out
of the figure above so that the overall trend could be seen more
clearly. The figure below does show the year to year variation
(dotted black line) and
the uncertainties (green bars, note how the uncertainty is lower in
recent years) in the yearly measurements.
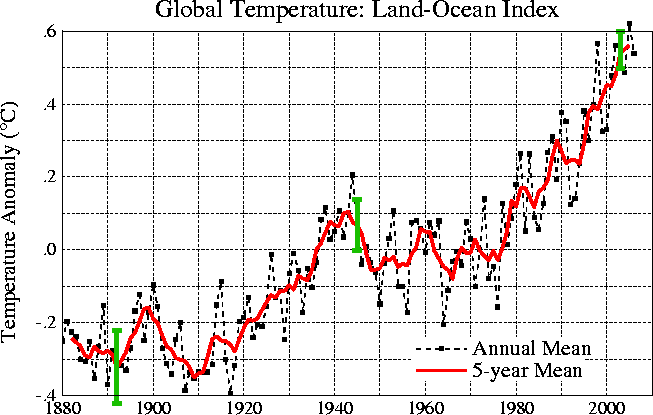
These data are from the NASA Goddard
Institute for Space Studies site.
Temperatures here are
compared to the 1951-1980 mean. Temperatures prior to about 1930
were colder than the 1951-1980 mean and temperatures after 1980 were
warmer.
Here's another plot of global temperature change over a
slightly longer
time period from the University
of East
Anglia Climatic Research Unit
2nd part
Now it would be interesting to
know how temperature was changing prior
to the mid-1800s. This is similar to what happened when the
scientists wanted to know what carbon dioxide concentrations looked
like prior to 1958. In that case they were able to go back and
analyze air samples from the past (air trapped in bubbles in ice
sheets).
That doesn't work with temperature.
Imagine putting some air in a bottle, sealing the bottle, putting the
bottle on a shelf, and letting it sit for 100 years. In 2108 you
could take the bottle down from the shelf, carefully remove the air,
and measure
what the CO2 concentration in the air had been in 2008 when the air was
sealed in the bottle. You couldn't, in 2108, use the air in the
bottle to determine what the temperature of the air was when it was
originally put into the bottle in 2008.
With temperature you need to use
proxy data.
You need to look for something else whose presence, concentration, or
composition depended on
the temperature at some time in the past.
Here's a proxy data example.
Let's say you want
to determine how many students are living in
a house near the university.
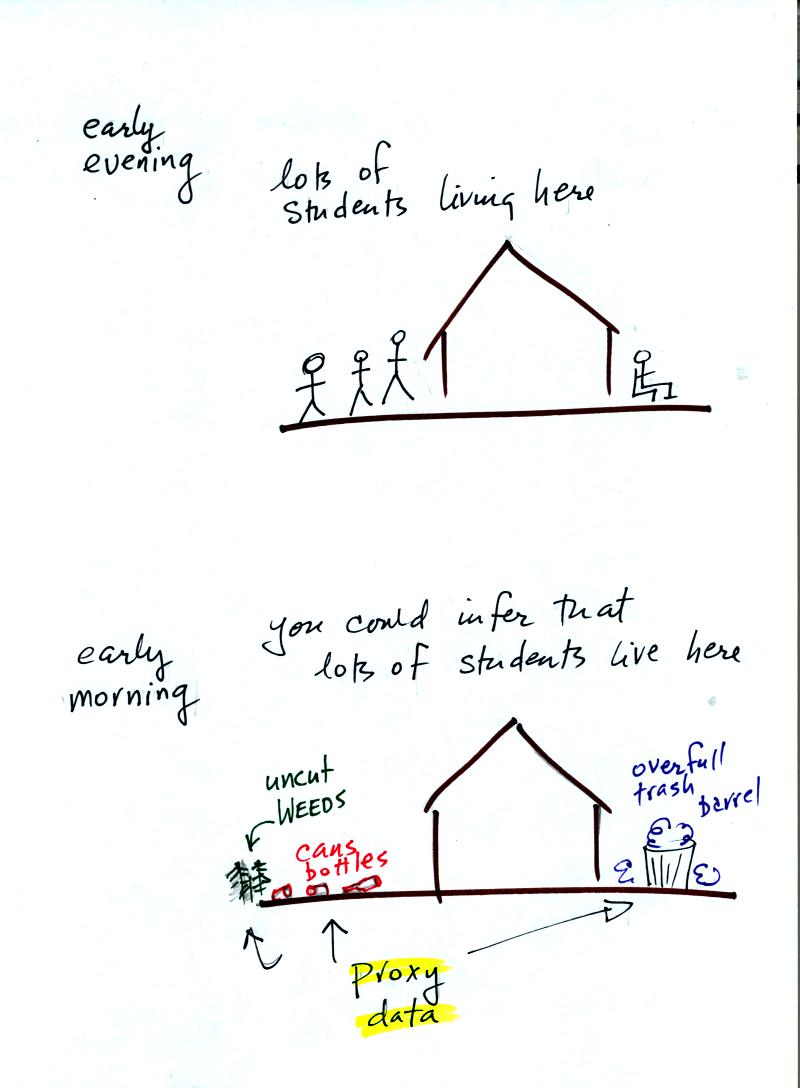
You
could walk by the house late in
the afternoon when the students might be outside and count them.
That
would be a direct measurement (this would be like measuring temperature
with a thermometer). There could still be some errors in your
measurement (some students might be inside the house and might not be
counted, some of
the people outside might not live at the house).
If you were to walk by early in the morning it is likely that the
students would be inside sleeping (or in one of the 8 am NATS 101
classes). In that case you might
look for other clues (such as the number of empty bottles in the yard)
that might give you an idea of how many students
lived in that house. You would use these proxy data to come up
with an estimate of the number of students inside the house.
In the case of temperature scientists look at a variety of
things. They could look at tree rings. The width of each
yearly ring depends on the depends on the temperature and
precipitation at the time the ring formed. They analyze
coral. Coral is made up of calcium carbonate, a molecule that
contains oxygen. The relative amounts of the oxygen-16 and
oxygen-18
isotopes depends
on the temperature that existed at the time the coral grew.
Scientists can analyze lake bed and ocean sediments. The types
of plant and animal fossils that they find depend on
the water temperature at the time. They can even use the ice
cores. The ice, H2O, contains oxygen and the relative
amounts of
various oxygen isotopes depends on the temperature at the time the ice
fell from the sky as snow.
Here's an idea of how oxygen isotope data can be used to determine past
temperature.

The two isotopes
of
oxygen contain different numbers of neutrons in their
nuclei. Both atoms have the same number of protons.
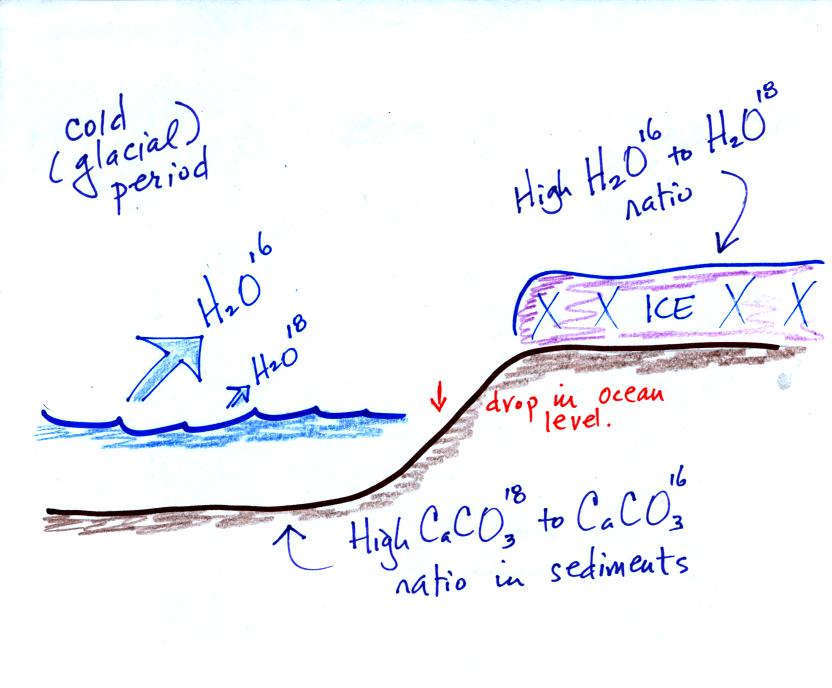
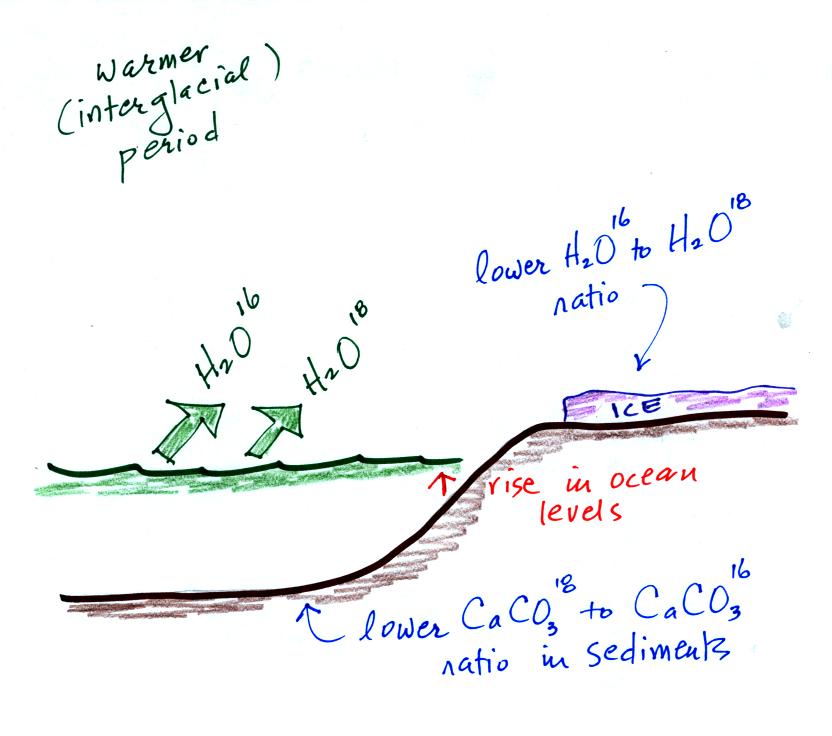
During a cold period, the H2O16 form of water
evaporates more rapidly
than the H2O18 form. You would find
relatively large
amounts of O16 in glacial ice. Since most of the H2O18
remains in
the ocean, it is found in relatively high amounts in calcium carbonate
in ocean sediments.
The reverse is true during warmer periods.
Using proxy data
scientists have been able to estimate average
surface temperatures for 100,000s of years into the past. The
next figure (bottom of p. 3 in the photocopied Classnotes) shows what
temperature has been doing since 1000 AD.
This is for the northern hemisphere only, not the globe.
The
blue
portion of the figure shows the estimates of temperature (again
relative to the 1961-1990 mean) derived from proxy data. The red
portion is the instrumental
measurements made between about 1850 and the present day. There
is also a lot of year
to year variation and uncertainty that is not shown on the figure
above.
Many scientists would argue that this graph is strong support of a
connection between rising atmospheric greenhouse gas concentrations and
global warming. Early in this time interval when CO2
concentration was constant, there is little temperature change.
Temperature only begins to rise in about 1900 when we know an increase
in atmospheric carbon dioxide concentrations was underway.
There is historical evidence in Europe of a medieval warm period
lasting from 800 AD to - 1200 AD or so and a cold period, the "Little
Ice Age, " which lasted from about 1400 AD to the mid 1800s.
These are not clearly apparent in the temperature plot above.
This leads some scientists to question the validity of this temperature
reconstruction. Scientists also suggest that if large changes in
climate such as the Medieval warm period and the Little Ice Age can
occur naturally, then maybe the warming that is occurring at the
present time also has a natural cause.
Here's the figure that the sketch above was based on

Climate
Change 2001 - The Scientific Basis
Contribution of Working Group I to the 3rd Assessment Report of the
Intergovernmental Panel on Climate Change (IPCC)
Here's a comparison of several
additional estimates of
temperature changes
over the past 1000 years or so

This is from the
University of
East Anglia Climatic Research Unit again. Some of these
curves do show a little bit more temperature variation between 1000 AD
and 1900 AD than the hockey stick plot above.
That is
where we will leave this topic for now, we've only covered a small part
of a large debate.
SUMMARY
There is general agreement that
Atmospheric CO2 and other greenhouse gas
concentrations are
increasing and that
The earth is warming
Not everyone agrees
on the Causes (natural or manmade) of the warming,
how much additional warming there will be, or
on the Effects that warming will have on weather and
climate in the years to come