Monday Sept. 15, 2008
Click here for a more printer friendly
version of these notes in Microsoft WORD format.
All this week in NATS 101 we'll be featuring a local band, Calexico,
and Luz de Luna, a Tucson Mariachi band. Today you heard "The Ballad of Cable
Hogue" and "Si
Tu Disais." Calexico and Luz de Luna were appearing at the
Barbican Theater in London and were joined by a French singer named
François Breut. Incidentally Calexico (and Luz de Luna,
perhaps) will be appearing at the Rialto Theater in
Tucson this coming Saturday night.
The Practice Quiz and an In-class
Optional Assignment from last Friday were returned in class today.
The 1S1P Bonus Assignment is due on
Wednesday (Sep. 17).
An Optional homework Assignment was
distributed in class and will be due next Monday. You should
complete this assignment before coming to class and have it ready to
turn in at the beginning of class. You can earn extra credit by
completing and turning in optional assignments like this.
Finally, 1S1P Assignment #1 has been posted
on the class webpage. Reports are due on or before Wed. Oct. 1.

Sea level pressures
usually fall between 950 mb and 1050 mb.
Record high sea level
pressure values occur during cold weather.
Record low pressure
values have all been set by intense hurricanes (the record setting low
pressure is the reason these storms were so intense). Hurricane
Wilma in 2005 set a new record low sea level pressure reading for the
Atlantic. Hurricane Katrina had a pressure of 902 mb.
You'll find a list of the most intense, destructive, and deadly
hurricanes on p. 146a in the photocopied ClassNotes.

Air pressure is a force that pushes
downward, upward, and
sideways.
If you fill a balloon with air and then push downward on it, you can
feel the air in the balloon pushing back (pushing upward). You'd
see the air in the balloon pushing sideways as well.
The air
pressure in the four tires on your automobile pushes down on the road
(that's something you would feel if the car ran over your foot) and
pushes upward
with enough force to keep the 1000 or 2000 pound vehicle off the
road.

A "people pyramid" might help you
to understand what is going on in the atmosphere (the picture above wasn't shown in class).
If the bottom person in the stack above were standing on a scale, the
scale would measure the total weight of all the people in the
pile. That's analogous to sea level pressure being determined by
the weight of the atmosphere above. The bottom person in the
picture above must be strong enough to support the weight of all the
people above. That equivalent to the bottom layer of the
atmosphere having enough pressure, pressure that points up down and
sideways, to support the weight of the air above.
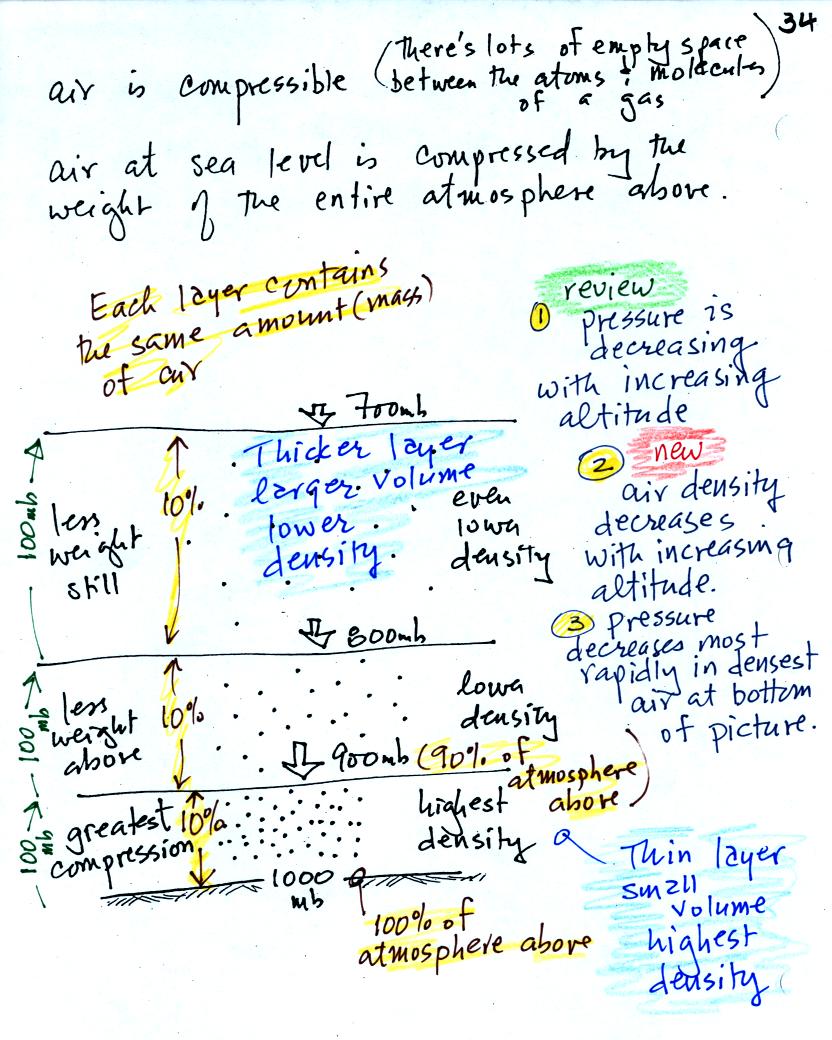
In class on Friday we used a stack of bricks to try to understand that
pressure at any level in the atmosphere is determined by the weight of
the air overhead. Now we will imagine a stack of matresses
to
understand why air density decreases with increasing altitude.
This is a more carefully drawn
version of what was shown in class. Mattresses are
compressible. The mattress at the
bottom of the
pile is compressed the most by the weight of all the mattresses
above. The mattresses higher up aren't squished as much because
their
is less weight remaining above.
In the case of the atmosphere layers of air behave in just the same way
as matresses.
There's a lot of information in this figure. It is worth
spending a minute or two looking at it and thinking about it.
1. You can first notice and remember that pressure decreases
with increasing altitude.
Each layer of air contain the same amount (mass) of air. You can
tell because the pressure decrease as you move upward through each
layer is the same (100 mb).
2. The densest air is found in the bottom layer because the air is
squeezed into a smaller volume than the other layers. Air density
decreases with increasing altitude.
3. You again notice something that we covered earlier: the most rapid
rate of pressure decrease with increasing altitude is in the densest
air in the bottom air layer.
We took a little detour at this point.
Hot air balloons can go up or down. Most everyone in the
classroom knows that gravity is the force that would cause a hot air
balloon to sink. Nobody was willing to suggest a force that might
cause a hot air balloon to rise. We'll come back to this picture
later.
Class continued with a demonstration of the upward force
caused by air
pressure.
The demonstration is summarized on p. 35a in the photocopied Classnotes.
Here's a little bit more detailed and more complete explanation of
what is going on. First the case of a water balloon.

In the demonstration a wine glass is filled with water. A small
plastic lid is used to cover the wine glass. You can then turn
the glass upside down without the water falling out.

The demonstration was repeated using a 4 Liter flash (more than a
gallon of water, more than 8 pounds of water). The upward
pressure force was still able to keep the water in the flask (much of
the weight of the water is pushing against the sides of the flask which
the instructor was supporting with his arms).
Look back at the hot air balloon. Can you now think of an
upward force that might cause the balloon to rise?
So far we
have looked at how pressure and air density change with increasing
altitude. Now we will look how air temperature changes with
altitude.
Here's the figure we drew in class.
The atmosphere can be split
into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. There are
additional layers (the mesosphere and the thermosphere) above 50 km but
we won't worry about them. The
numbered points were added after class.
1. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature usually decreases
with
increasing altitude. [the troposphere is usually a little higher
in the tropics and lower at polar latitudes]
The troposphere contains most of the water vapor
in the atmosphere (the water vapor comes from evaporation of ocean
water) and is
where most of the clouds and weather occurs. The
troposphere can be stable or unstable (tropo means to turn over and
refers to the fact that air can move up and down in the
troposphere).
2a. The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are occurring. When the thunderstorm reaches the
top of the troposphere, it runs into the stable stratosphere. The
air can't continue to rise into the stable stratosphere so the cloud
flattens out and forms an anvil (anvil is the name given to the flat
top of the thunderstorm, I might
have forgotten to mention that term in class). The
flat anvil top is something
that you can go outside and see and often marks the top of the
troposphere.
2b. The summit of Mt. Everest is a little over 29,000
ft. tall and is
close to the top of the troposphere.
2c. Cruising altitude in a passenger jet is usually between
30,000 and 40,000, near or just above the top of the troposphere.
3. Temperature remains constant between 10 and 20 km
and then
increases with increasing altitude between 20 and 50 km. These
two sections form the stratosphere. The stratosphere is a
very stable air layer. Increasing temperature with increasing
altitude is called an
inversion. This is what makes the stratosphere so stable.
4. A kilometer is one
thousand meters. Since 1 meter is about 3 feet, 10 km is about
30,000 feet. There are 5280 feet in a mile so this is about 6
miles.
There were a few more points to make, but we ran out of time. The
following figure wasn't shown in class.
5. Sunlight is a mixture of ultraviolet,
visible, and
infrared light. We can see the visible light.
5a. Much of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air just above. As you get further and
further from the warm ground,
the air is
colder and colder. This
explains why air temperature decreases with increasing altitude.
5b. How do you explain increasing temperature with
increasing
altitude in the stratosphere.
The ozone layer is found in the stratosphere
(peak concentrations are found near 25 km altitude). Absorption
of
ultraviolet light by ozone warms the air in the stratosphere and
explains why the air can warm. The air in the stratosphere is
much less dense (thinner) than in the troposphere. It doesn't
take as much energy to warm this thin air as it would to warm denser
air closer to the ground.
6. That's a manned
balloon; Auguste Piccard and Paul Kipfer are
inside. They were to first men to travel into the
stratosphere (see pps 31 & 32 in
the photocopied Class Notes). We'll see a short video showing
part of their adventure at some point in the next week or
so.