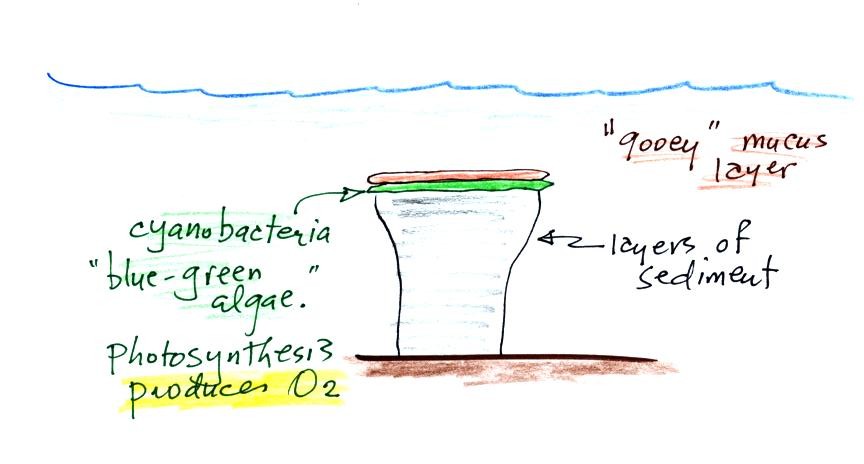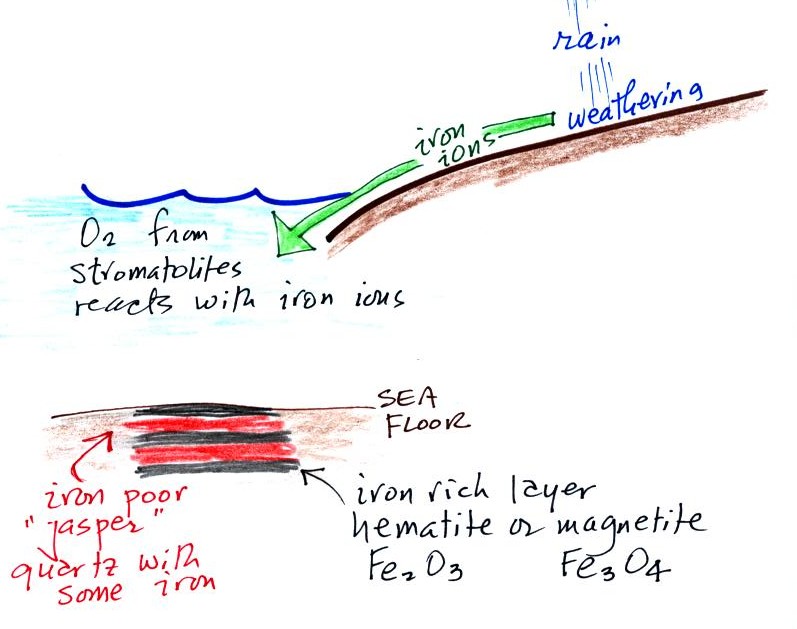
Periodically the oxygen production would
decrease or stop (rising oxygen levels might have killed the
cyanobacteria or seasonal changes in incoming sunlight might
have slowed the photosynthesis). During these times of
low oxygen concentration, red layers of jasper would form on
the ocean bottom. Eventually the cyanobacteria would
recover, begin producing oxygen again, and a new layer of
hematite or magnetite would form. The rocks that
resulted, containing alternating layers of black hematite or
magnetite and red layers of jasper are known as the banded
iron formation. In addition to the
red and black layers, you see yellow layers made of fibers of
quartz in the samples passed around class. The
rocks are fairly heavy because they contain a lot of iron, but
the most impressive thing about them in my opinion is their
age - they are a few billion years old!
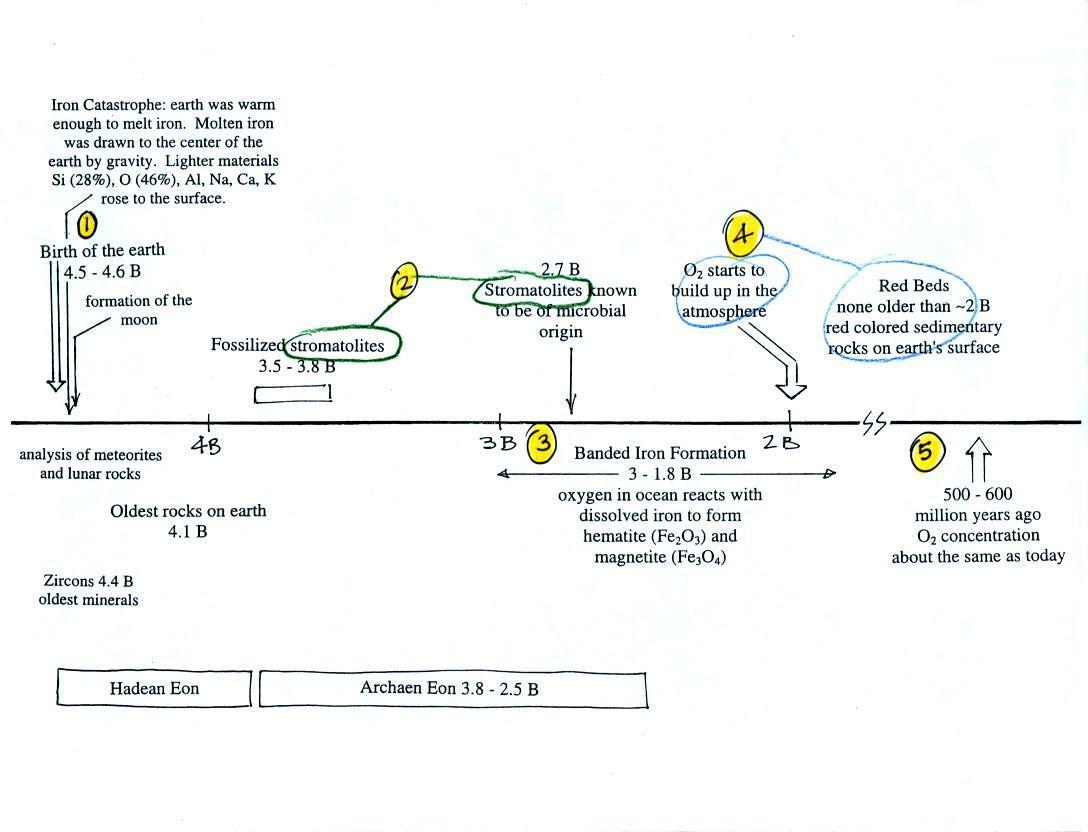
Eventually the oxygen in the ocean reacted with all of the
iron ions and was free
to move from the ocean into the atmosphere. Once in the
air, the oxygen could react with iron in sediments on the
earth's surface. This produced red colored (rust
colored) sedimentary rock. These are called "Red Beds" (Point 4). None of
these so-called red beds are older than about 2 B years
old. Thus it appears that a real buildup up of oxygen in
the atmosphere began around 2 B years ago. Oxygen
concentrations reached levels that are about the same as today
around 500 to 600 million years ago (Point 5 in the figure).
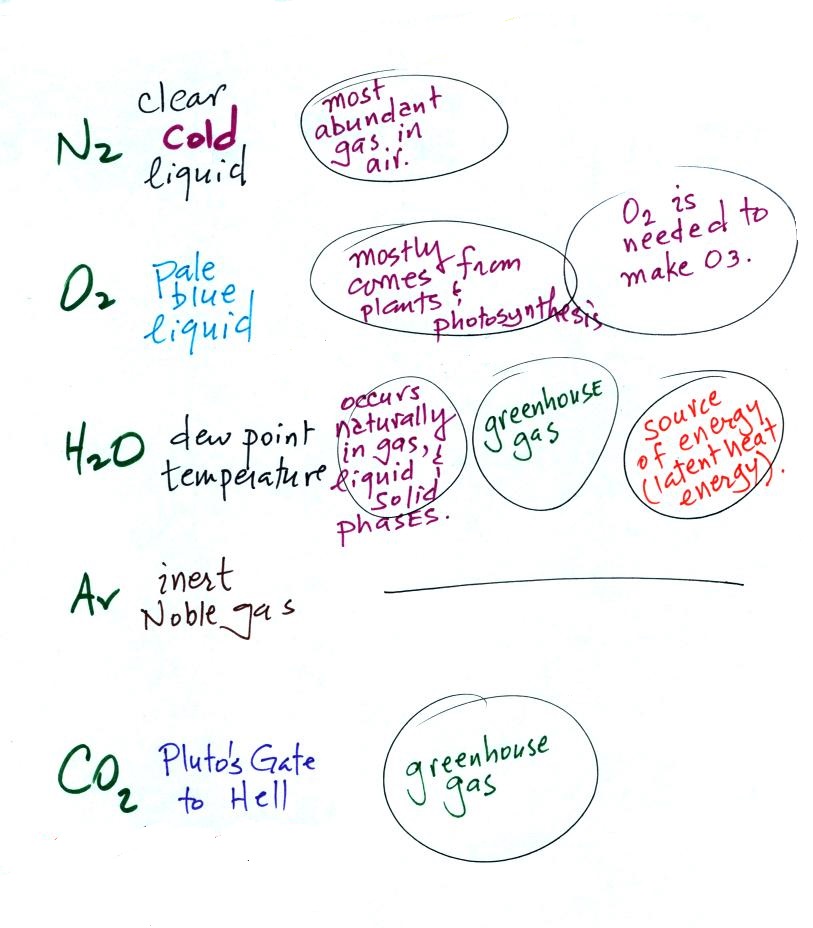
Below is a more carefully drawn version of a figure drawn
in class. At the top of the list below are the 5 most
abundant gases in the atmosphere (something you're seeing for
the 4th or 5th time). Several more important trace
gases were added to the bottom of the
figure. Trace gases are gases found in low
concentrations (and often the concentrations vary with time
and location). Low concentrations doesn't mean they
aren't important, however.
Carbon monoxide, nitric oxide, nitrogen dioxide,
ozone, and sulfur dioxide are some of the major air
pollutants. We'll cover 3 of these in more detail next
week.
Water vapor, carbon dioxide, methane, nitrous oxide (N2O = laughing gas),
chlorofluorocarbons, and ozone are all greenhouse
gases. Increasing atmospheric concentrations of these
gases are responsible for the current concern over climate
change and global warming. We'll discuss this topic
and learn more about how the greenhouse effect actually
works later in the course.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i)
Ozone in the stratosphere (a layer of the atmosphere between
about 10 and 50 km altitude) is beneficial because it
absorbs dangerous high energy ultraviolet (UV) light coming
from the sun. Without the protection of the ozone
layer, life as we know it would not exist on the surface of
the earth. It was only after ozone started to buildup
in the atmosphere that life could move from the oceans onto
land. Chlorofluorocarbons are of concern in the
atmosphere because they destroy stratospheric ozone.
(ii)
In the troposphere (the bottom 10 kilometers or so of the
atmosphere and where we live) ozone is a pollutant and is
one of the main ingredients in photochemical smog.
(iii) Ozone is also
a greenhouse gas.
We'll go back to our list of the
5 main gases in the atmosphere one last time and add some more
information about water vapor and carbon dioxide.
Water vapor and carbon dioxide are probably the best known
of the greenhouse gases. We'll cover the greenhouse
effect, climate change and global warming later in the
semester. The fact that water vapor is a
source (an important source) of latent heat energy is
something else we'll cover later in the semester.
Finally,
I wasn't being entirely
honest when I said that gases are invisible.
Some gases can be seen, here are some examples.
 |
|
 |
|
Bromine in
both liquid and gaseous phases. Bromine and mercury
are the only two elements that exist as liquids at
room temperature. The bromine is in a sealed
glass ampoule inside an acrylic cube. Bromine
could be safely brought to class in a container like
this.
This photo was taken by Alchemist-hp and was
Picture of the Day on the English Wikipedia on Oct. 29,
2010.
|
Chlorine (Cl2)
I found this image here
|
Iodine
Also an element that is normally found in solid
form. The solid sublimates, i.e. it changes
directly from solid to gas (you would probably need to
heat the solid iodine to produce gas as dense as seen
in the picture above). source
of this image
|
Nitrogen dioxide (NO2)
An important pollutant.
source
of this image
|