Tuesday Sep. 17, 2013
With the final episode approaching, it seemed like some music
from Breaking Bad might be appropriate: You heard Los
Cuates de Sinaloa "Negro Y Azul",
Los Zafiros "He
Venido", Cumbre Norteno "Simplemente
Amame", Rodrigo y Gabriela "Tamacun".
I only started watching the show in the last year and am
mid way through season 3.
The 1S1P Assignment #1
reports were collected today. We'll get started on those
this week, but it will take some time to get them all
graded.
The Bonus Assignment
report is due on Thursday.
The Practice Quiz has been graded and was returned in class
today. The average grades (both the 8 am and 9:30 sections
are shown below) were similar to grades from previous
classes. You'll find answers
to the questions on the Practice Quiz online.
An Optional
(Extra Credit) Assignment was handed out in class. The
assignment is due at the start of class on Thursday.
|
T Th
(9:30 am) class
|
T Th
(8 am) class
|
13
|
67%
|
63%
|
|
MWF (2 pm) class
|
T Th (8 am) class
|
F12
|
66%
|
66%
|
F11
|
65%
|
65%
|
F10
|
60%
|
67%
|
F09
|
66%
|
68%
|
F08
|
64%
|
65%
|
What difference does it make if pressure decreases with
increasing altitude or if pressure pushes upward, downward, and
sideways?
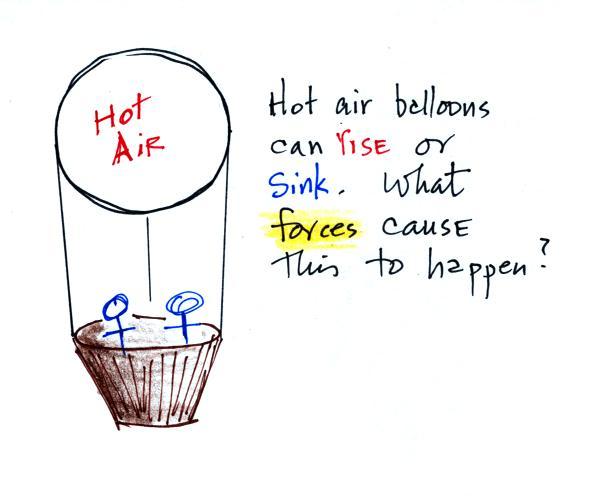
Hot air balloons can go up and come back down. I'm pretty
sure you know what would cause the balloon to sink. I
suspect you don't know what causes it to float upward.
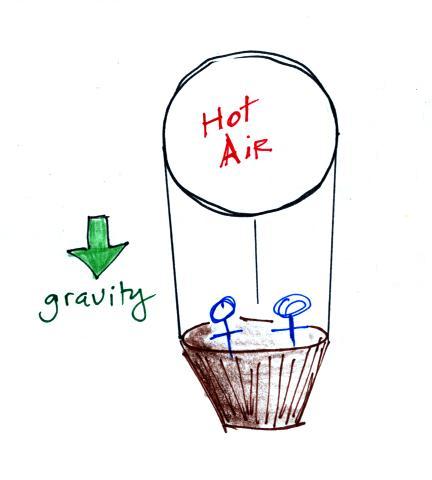
Gravity pulls downward on the
balloon. The strength of this force will depend on whether
the air is hot low density air (light weight) or cold higher
density air (heavier air).
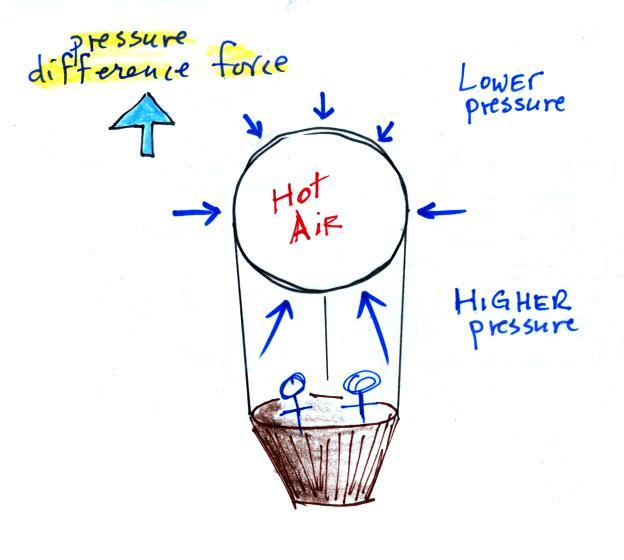
Pressure from the air surrounding the balloon is pushing
against the top, bottom, and sides of the balloon (the blue arrows
shown above at right). Pressure decreases with
increasing altitude. The pressure at the bottom pushing up
is a little stronger than the force at the top pushing down (the
pressures at the sides cancel each other out). Decreasing
pressure with increasing altitude creates an upward pointing
pressure difference force that opposes gravity. It is this
upward pressure difference force that can cause a balloon to float
upward.
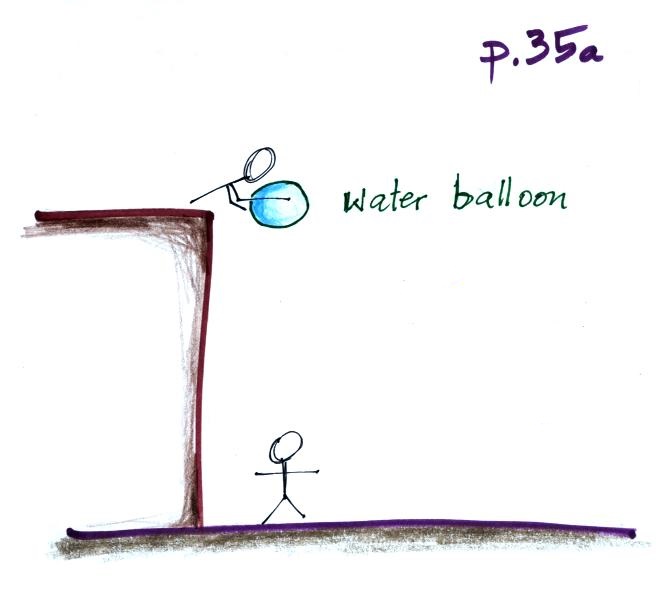
This was a logical point to do a demonstration. A demo
that tries to prove that air pressure really does push upward as
well as downward. Not only that but that the upward force is
fairly strong. The demonstration is summarized on p. 35a in
the ClassNotes.
It's pretty obvious that if you
fill a balloon with a little water and let go it will
drop. The upward pressure difference force is present but
is much weaker than gravity.
Here's a little bit more
detailed and more complete explanation of what is going on.

The figure at left shows air pressure (red arrows) pushing on
all the sides of the balloon. Because pressure decreases
with increasing altitude, the pressure from the air at the top
of the balloon pushing downward (strength=14) is a little weaker
than the pressure from the air at the bottom of the balloon that
is pushing upward (strength=15). The two sideways forces
cancel each other out. The total effect of the pressure is
a weak upward pressure difference force (1 unit of upward force
shown at the top of the right figure).
Gravity exerts a downward force on the water balloon. In
the figure at right you can see that the gravity force
(strength=10) is stronger than the upward pressure difference
force (strength=1). The balloon falls as a result.
This is what you know would happen if you let go of a water
balloon, it would fall.
In the demonstration a wine glass is filled with water (about
the same amount of water that you might put in a small water
balloon).
A small plastic lid is used to cover the wine glass (you'll
need to look hard to see the lid in the photo above). The
wine glass is then turned upside and the water does not fall
out. The water was colored red in the class demonstration.

All the same forces are shown again in the left most
figure. In the right two figures we separate this into two
parts - a water and lid part and an empty glass part. First
the water inside the glass isn't feeling the downward and sideways
pressure forces because they're pushing on the glass and I was
holding onto the glass.
Gravity still pulls downward on the water with the same 10 units
of force. But the upward pressure force is able to overcome
the downward pull of gravity. It can do this because all 15
units are used to overcome gravity and not to cancel out the
downward pointing pressure force. The net upward force is
strong enough to keep the water in the glass.
The demonstration was repeated using a 4 Liter flash (more than
a gallon of water, more than 8 pounds of water). The upward
pressure force was still able to keep the water in the flask (much
of the weight of the water is pushing against the sides of the
flask which the instructor was supporting with his arms).
We've spent a lot of time looking at
air pressure and how it changes with altitude. Next
we'll consider air density and air temperature.
How does air density change with increasing
altitude? You should know the answer to that
question. You get out of breath more
easily at high altitude than at sea level. Air
gets thinner (less dense) at higher altitude. A
lungful of air at high altitude just doesn't contain as
much oxygen as at lower altitude or at sea level.
We've used bricks to try to
understand that air pressure depends on the weight of the
air overhead and that it decreases with increasing
altitude. Because air is compressible, a stack of
mattresses might be a more realistic representation of
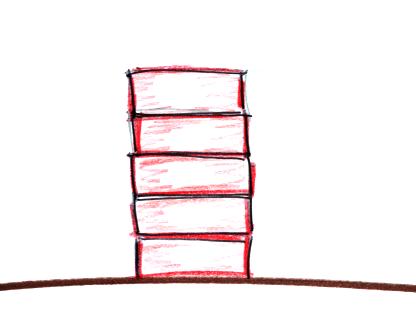
layers of air than a pile of bricks.
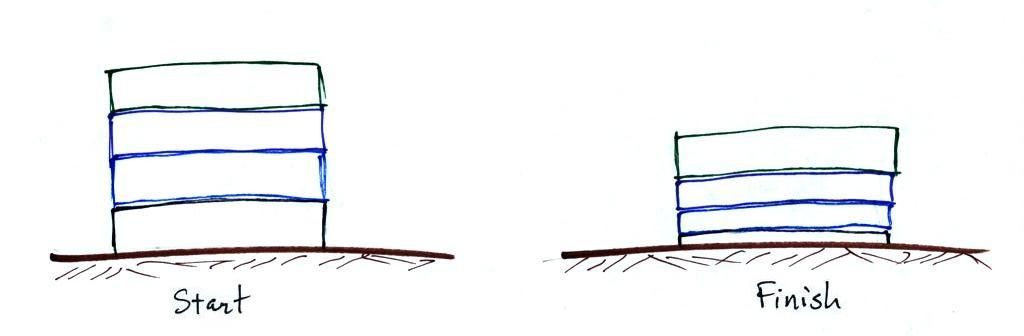
Four mattresses are stacked on top of
each other. Mattresses are reasonably heavy, the
mattress at the bottom of the pile is compressed by the
weight of the three mattresses above. This is shown
at right. The mattresses higher up aren't squished
as much because their is less weight remaining
above. The same is true with layers of air in the
atmosphere.
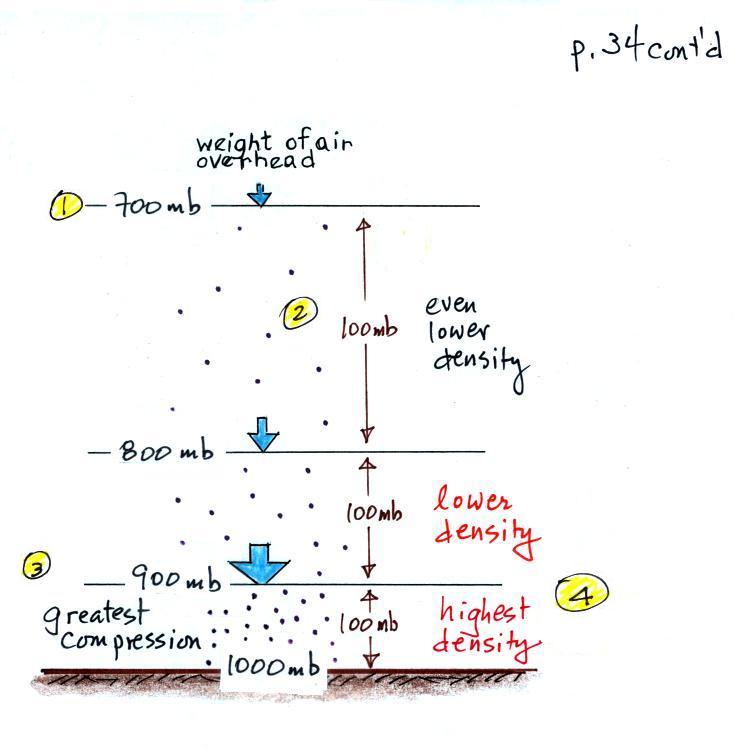
The statement above is at the top of p. 34 in the
photocopied ClassNotes. I've redrawn the figure
found at the bottom of p. 34 below.
There's a surprising amount of information in this figure
and it is worth spending a minute or two looking for it
and thinking about it.
1. You can first notice and remember that pressure
decreases with increasing altitude. 1000 mb at the
bottom decreases to 700 mb at the top of the
picture. You should be able to explain why this
happens.
2. Each layer of air contain the same amount
(mass) of air. This is a fairly subtle point.
You can tell because the pressure drops by 100 mb as you
move upward through each layer. Pressure depends on
weight. So if all the pressure changes are equal,
the weights of each of the layers must be the same.
Each of the layers must contain the same amount (mass) of
air (each layer contains 10% of the air in the
atmosphere).
3. The densest air is found at the bottom of the
picture. The bottom layer is compressed the most
because it is supporting the weight of all of the rest of
the atmosphere. It is the thinnest layer in the
picture and the layer with the smallest volume.
Since each layer has the same amount of air (same mass)
and the bottom layer has the smallest volume it must have
the highest density. The top layer has the same
amount of air but about twice the volume. It
therefore has a lower density (half the density of the air
at sea level). Density is decreasing with
increasing altitude.
4. Finally pressure is decreasing most rapidly with
increasing altitude in the densest air in the bottom
layer. This is something we covered last week and
something we'll use again 2 or 3 times later in the
semester.
What happens to air temperature with increasing
altitude. Again our personal experience is that it
decreases with increasing altitude. It is colder at
the top of Mt. Lemmon than it is here in the Tucson
valley.
That is true up to an altitude of about 10 km (about
30,000 ft.). People were very surprised in the early
1900s when they used balloons to carry instruments above
10 km and found that temperature stopped decreased and
even began to increase with increasing altitude.
Measurements of air temperature at high
altitude in unmanned balloons lead to the discovery of the
stratosphere in about 1900 (the information above is on p.
31 in the ClassNotes).
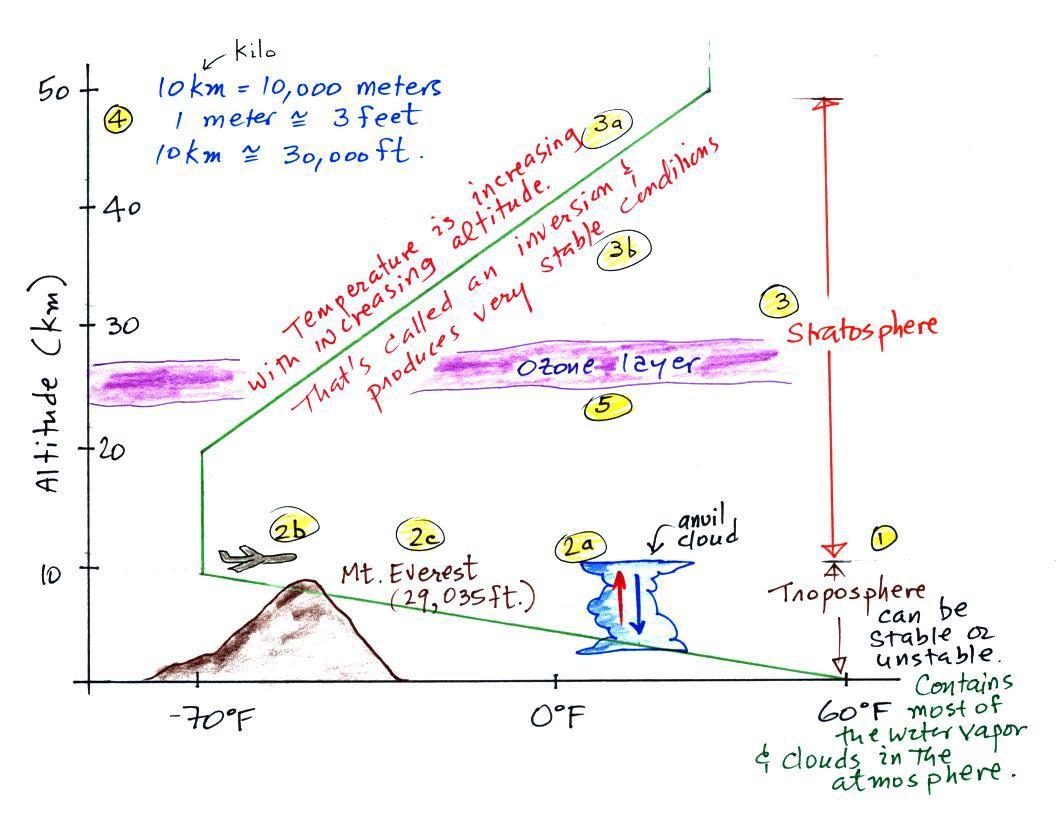
The figures below are more clearly drawn versions of
what was done in class.
The atmosphere can be split into layers depending on
whether temperature is increasing or decreasing with
increasing altitude. The two lowest layers are shown
in the figure above. There are additional layers
(the mesosphere and the thermosphere) above 50 km but we
won't worry about them in this class.
1. We live in the troposphere. The
troposphere is found, on average, between 0 and about 10
km altitude, and is where temperature usually decreases
with increasing altitude. [the troposphere is
usually a little higher in the tropics and lower at polar
latitudes]
The troposphere can be stable or unstable (tropo means
"to turn over" and refers to the fact that air can move up
and down in the troposphere). The troposphere
contains most of the water vapor in the atmosphere (the
water vapor comes from evaporation of ocean water and then
gets mixed throughout the troposphere by up and down air
motions) and is where most of the clouds and weather
occurs.
2a. The thunderstorm shown in the figure with
its strong updrafts and downdrafts indicates unstable
conditions. When the thunderstorm reaches the top of
the troposphere, it runs into the bottom of the
stratosphere which is a very stable layer. The air
can't continue to rise into the stratosphere so the cloud
flattens out and forms an anvil (anvil is the name given
to the flat top of the thunderstorm). The flat
anvil top is something that you can go outside and see and
often marks the top of the troposphere.
Here are several images of thunderstorms and
anvil clouds taken from above, from the International
Space Station (all 3 images courtesy of the Image
Science and Analysis Laboratory, NASA Johnson Space Flight
Center, www.eol.jsc.nasa.gov)
This photo was selected as the Picture
of the Day on Wikipedia for Dec. 22, 2007. Photo credit:
Luca Galluzi www.galluzi.it
2b. The summit of Mt. Everest is a little over
29,000 ft. tall and is close to the average height of the
top of the troposphere.
2c. Cruising altitude in a passenger jet is
usually between 30,000 and 40,000, near or just above the
top of the troposphere, and at the bottom of the
stratosphere. The next time you're in an airplane
try to look up at the sky above. There's less air
and less scattering of light. As a result the sky is
a darker blue. If you get high enough the sky would
eventually become black.
3. Temperature remains constant between
10 and 20 km and then increases with increasing altitude
between 20 and 50 km. These two sections form the
stratosphere. The stratosphere is a very stable air
layer. Increasing temperature with increasing
altitude is called an inversion. This is what makes
the stratosphere so stable.
4. A
kilometer is one thousand meters. Since 1 meter is
about 3
feet, 10 km is about 30,000 feet. There are
5280 feet in a mile so this is about 6
miles (about is usually close enough in this
class).
5. The ozone layer is found in the
stratosphere. Peak ozone concentrations occur near
25 km altitude.
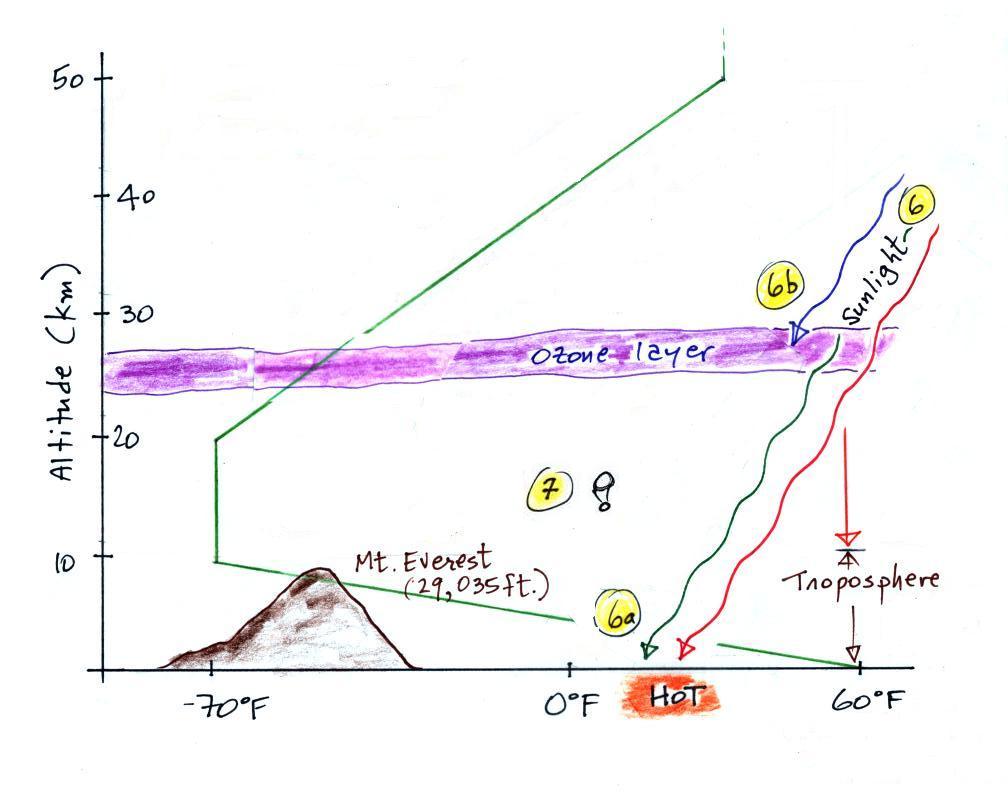
Here's the same picture drawn again (for clarity) with
some additional information. We need to explain why
when temperature decreases all the way up to the top of
the troposphere, it can start increasing again in the
stratosphere.
6. Sunlight is a mixture of ultraviolet (7%),
visible (44%, colored green in the picture above) and
infrared light (49%, colored red). We can see the
visible light.
6a. On average about 50% of the
sunlight arriving at the top of the atmosphere passes
through the atmosphere and is absorbed at the ground (20%
is absorbed by gases in the air, 30% is reflected back
into space). This warms the ground. The air in
contact with the ground is warmer than air just
above. As you get further and further from the warm
ground,
the air is colder and colder. This explains why air
temperature decreases with increasing altitude in the
troposphere.
5b. How do you explain increasing temperature
with increasing altitude in the stratosphere?
Absorption of ultraviolet light
by ozone warms the air in the stratosphere and explains
why the air can warm (oxygen also absorbs UV light).
The air in the stratosphere is much less dense (thinner)
than in the troposphere. So even though there is not
very much UV light in sunlight, it doesn't take as much
energy to warm this thin air as it would to warm denser
air closer to the ground.
7.
That's a manned balloon; Auguste
Piccard and Paul Kipfer are inside. They were the
first men to travel into the stratosphere (see pps 31
& 32 in the photocopied Class Notes). It really
was quite a daring trip at the time, and
they very nearly didn't survive it. More about
this later in class.
Pages 31 and 32 in the ClassNotes list some of the
significant events in the early study and exploration of
the atmosphere. A few of them are included below.
Note the mercury barometer was invented in
1643.
The earliest balloon trips into the upper
atmosphere were in unheated and unpressurized
gondolas. Climbers have made it to the summit of Mt.
Everest without carrying supplementary oxygen but it is
difficult and requires acclimation. Read "Into
Thin Air" by Jon Krakauer if you'd like to get some
idea of what it's like trying to climb Mt. Everest.
Note the clothing that Capt. Grey
had to wear to try to stay warm. All of his
trips were in an unpressurized open gondola.
This flight lead by Auguste
Piccard was the subject of a PBS program called
The Adventurers. A 10 minute segment from that
program was shown in class. I haven't been able
to find that video online.
Jacques
Piccard, Auguste's son, would later travel with
Lt. Don Walsh of the US Navy to a depth of about
35,800 feet in the ocean in the Mariana Trench.
They did that on Jan. 23, 1960.
Bertrand
Piccard, Jacques' son was part of the first two
man team to circle the globe non-stop in a balloon
(Mar. 20, 1999)
You might have
heard about Felix
Baumgartner and the Red Bull Stratos
balloon. On Oct. 14, 2012 he reached an altitude
of 128,177 feet and then jumped. He reached a
speed of 834 MPH on the way down (Mach 1.24 or 1.24
times the speed of sound). Here's a
video summary of the jump.