Acid Rain Demonstration
click here to download this in
a more printer friendly Microsoft WORD format
Some common acids are listed below. In solution the acid
molecules dissociate (split). The presence of H+
ions is what makes these materials acids.
Actually
for
a solution to be acidic it must have an H+ ion
concentration that is greater than the H+ ion
concentration found in distilled water. The H+
ion concentration in pure water is 10-7 moles
of H+ ions per liter of
water. A mole is
just a number, a very large number (6 x 1023).
It's the same idea as dozen. A dozen means you've got twelve
of something.
We often use the pH scale to measure acid concentration. An
H+ ion concentration of 10-7 moles/liter corresponds to
pH 7 (the pH value is computed by taking the -log10
of the H+
ion concentration).
A basic solution will have an H+
ion concentration that is lower than found in pure water.
Now we
can proceed to the demonstration. We will start with three
1000 mL beakers. They have all been filled with distilled
water. Some vinegar (contains acetic acid) has been added to
the left beaker. Some ammonia (a base) has been added to the right
beaker.
Then we add some bromothymol blue, a color indicator solution, to
all three beakers. Bromothymol blue has the amazing property
of changing color depending on whether it is mixed with an acid or
a base.

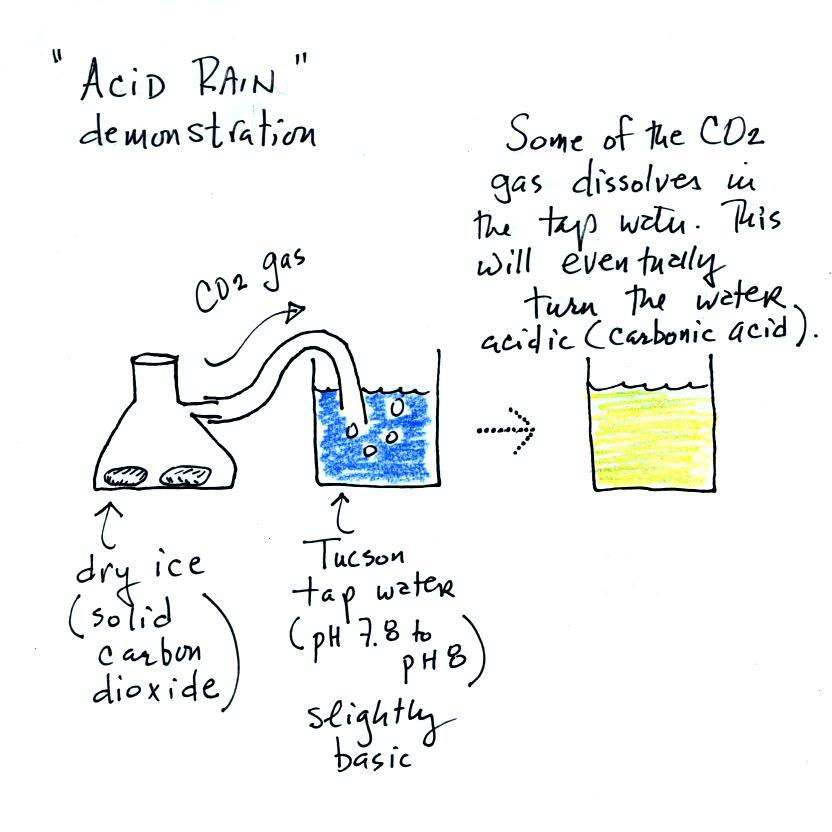
Dry ice sublimates. It turns directly from solid to ice
(ordinary ice melts and turns from solid to liquid). The
gaseous CO2 is invisible but
you can tell it is there because of the bubbles in the tap
water. Some of the CO2
dissolves as it bubbles through the water and slowly turns the
water acidic. You can tell that this is occurring because
the bromothymol blue indicator turns from deep blue to green and
eventually to yellow.

While we didn't actually produce acid rain, there is concern
that increasing atmospheric concentrations of carbon dioxide will
dissolve and acidify the world's oceans. This is discussed
in the following article from The Christian Science Monitor.
You can download a copy of the article here.
The main concern over increasing atmospheric carbon dioxide
concentrations is global warming from enhancement of the
greenhouse effect. We will discuss this topic in a few
weeks.






