Tues., Oct. 29
Girl In A Coma "As the World
Falls Down" (2:37), "Walkin After
Midnight" (2:49), "One Eyed Fool"
(3:17), "Smart"
(3:20), "Si
Una Vez" (3:11), "Come On Lets
Go" (2:11), "While My
Guitar Gently Weeps" (3:20)
Reports on two of the 1S1P
Assignment #2 topics were collected today (Ultraviolet light
and Global warming, melting ice, and sea level rise). There
is a 3rd topic (Koppen climate classification system) that isn't
due until next week. I've seen that some people are turning
in three reports, even though you are only supposed to do
two. I might give everyone that opportunity but will only
allow you up to half credit on the 3rd topic (half credit on the
3rd topic only if it's your 3rd report, if it's your 1st or 2nd
report you can still earn full credit).
There are also a couple of new Bonus
1S1P Assignment topics. You can do as many of those as
you'd like regardless of how many Assignment #2 reports you've
written.
There are a couple of Optional
Assignments still available. Both are due on Thursday
Oct. 31.
Midterm grade summaries were prepared over the weekend and were
handed out in class today. You'll find more information
embedded in today's notes.
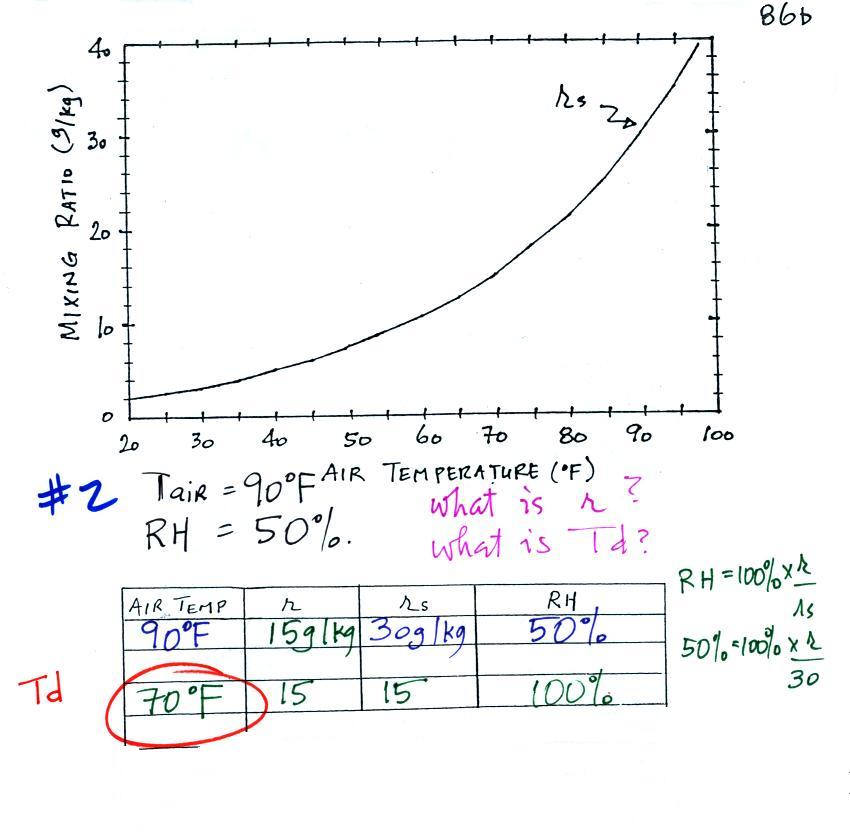
We have three more humidity example problems to do.
Example 2
We're given an air temperature of 90 F and a
relative humidity of 50%; we'll try to figure out the mixing ratio
and the dew point temperature. Here's something like what we
ended up with in class.
The problem is worked out in detail below:
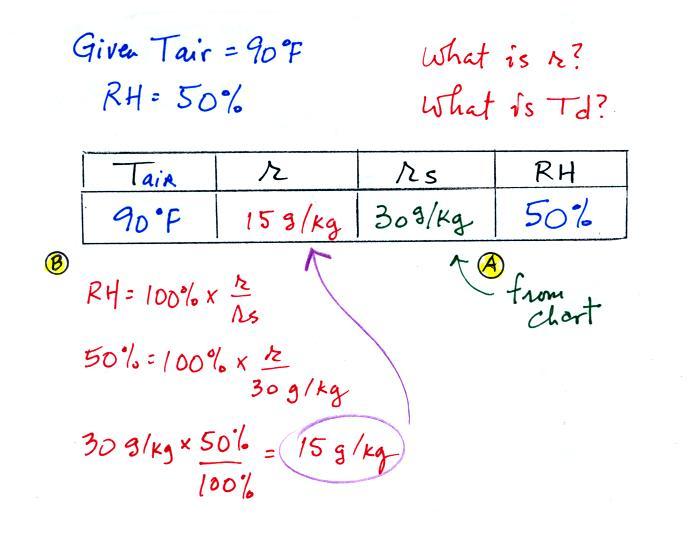
First you fill in the air temperature and the RH data
that you are given.
(A) since you know the air's temperature you can look up the
saturation mixing ratio (30 g/kg).
(B) Then you might be able to figure out the mixing ratio in
your head. Air that is filled to 50% of its capacity could
hold up to 30 g/kg. Half of 30 is 15, that is the mixing
ratio. Or you can substitute into the relative humidity
formula and solve for the mixing ratio. The details of that
calculation are shown above at B.
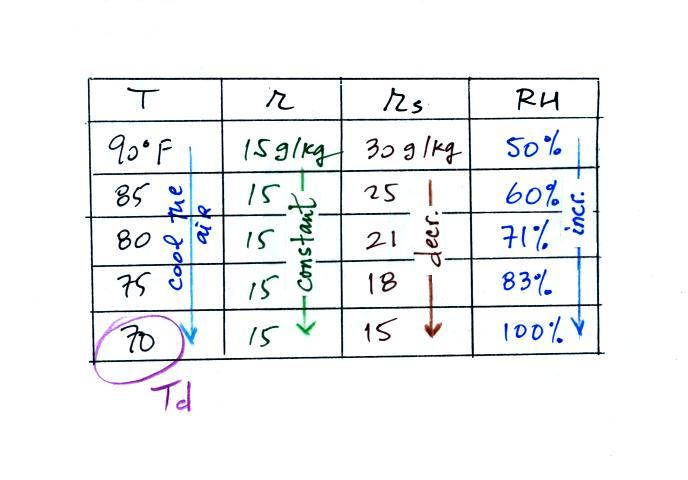
Finally you imagine cooling the air. Notice how the
saturation mixing ratio decreases, the mixing ratio stays
constant, and the relative humidity increases as the air is
cooled. In this example the RH reached 100% when the
air had cooled to 70 F. That is the dew point temperature.
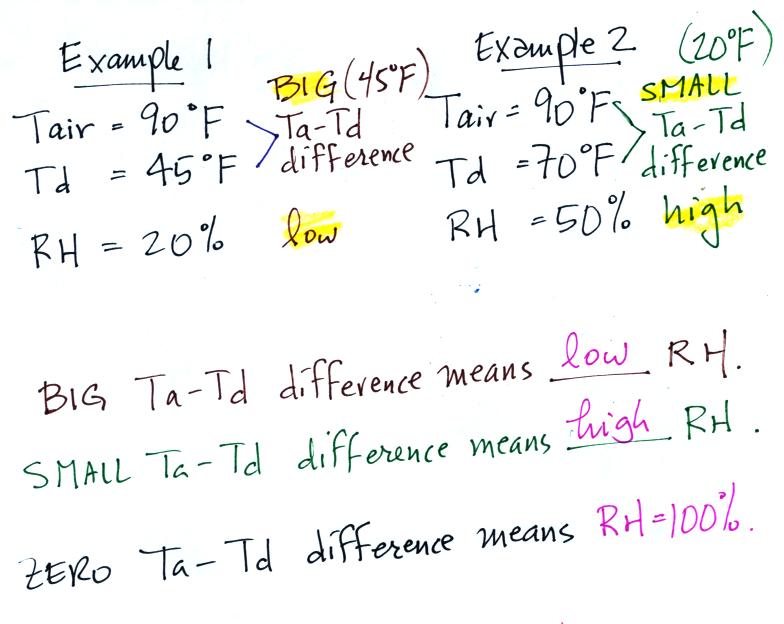
We can use results from humidity problems #1 and #2 to learn and
understand a useful rule.
In the first example the difference between the air and
dew point temperatures was large (45 F) and the RH was
low (20%).
In the 2nd problem the difference between the air and dew point
temperatures was smaller (20 F) and the RH was higher (50%).
The easiest way to remember this rule is to remember the case
where there is no difference between the air and dew point
temperatures. The RH then would be 100%.
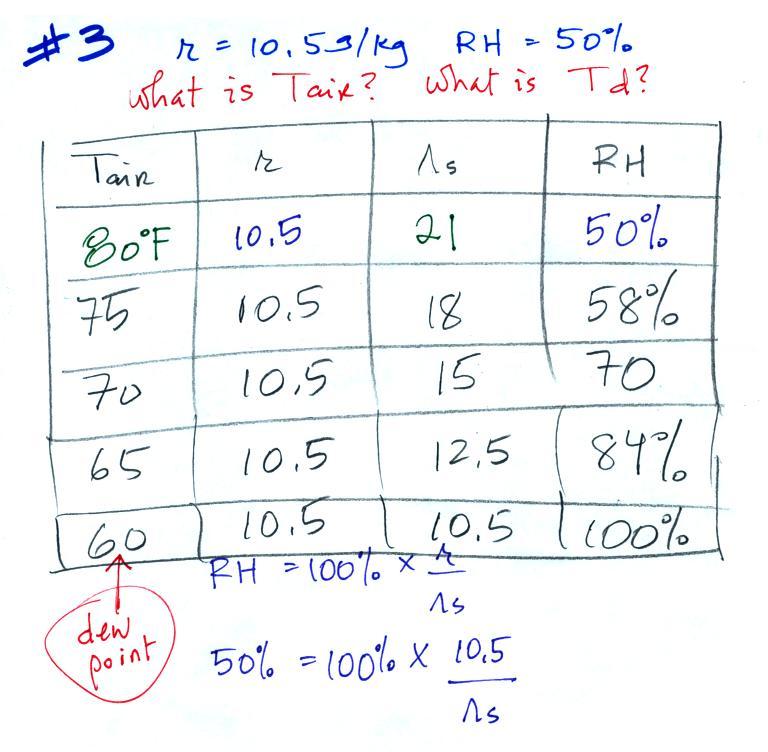
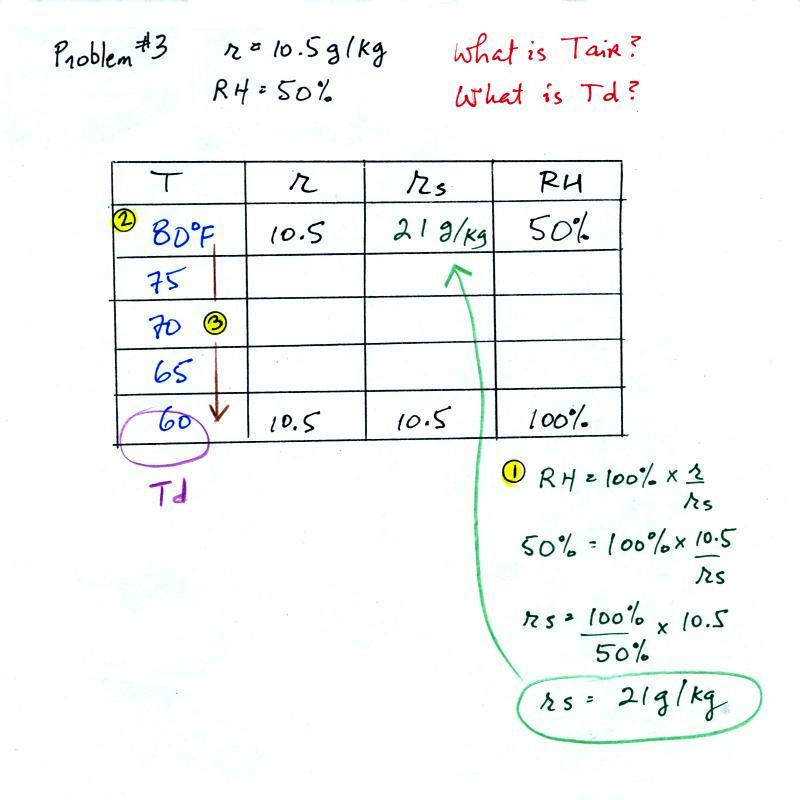
Example 3
You're given the the mixing ratio = 10.5 g/kg and a relative
humidity of 50%. You need to figure
out the air temperature and the dew point temperature.
Here's
the play by play solution to the question:
(1) The air contains 10.5 g/kg of water vapor. This is
50% (half) of what the air could potentially hold. So the
air's capacity, the saturation mixing ratio must be 21 g/kg (you
can either do this in your head or use the RH equation following
the steps shown above).
(2) Once you know the saturation mixing ratio you can look up the
air temperature in a table (80 F air has a saturation mixing ratio
of 21 g/kg)
(3) Then you imagine cooling the air until the RH becomes
100%. This occurs at 60 F. The dew point is 60 F.
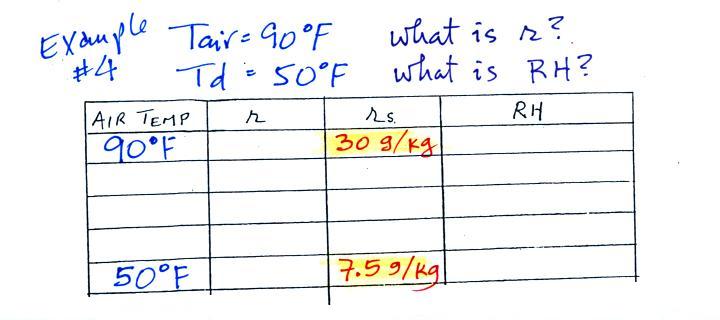
Example 4
Probably the most difficult problem of the bunch.
But one of the things we said about dew point is that it has the
same job as mixing ratio - it gives you an idea of the actual
amount of water vapor in the air. This problem will show
that if you know the dew point, you can quickly figure out the
mixing ratio. Knowing the dew point is equivalent to knowing
the mixing ratio.
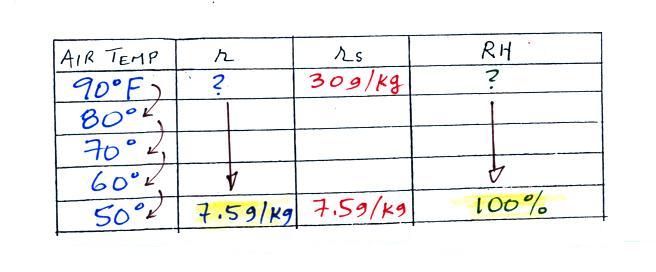
Here's what we ended up with in class, we were given the air
temperature and the dew point temperature. We were supposed
to figure out the mixing ratio and the relative humidity.
We enter the two temperatures onto a chart and look up the
saturation mixing ratio for each.
We ignore the fact that we don't know the mixing ratio.
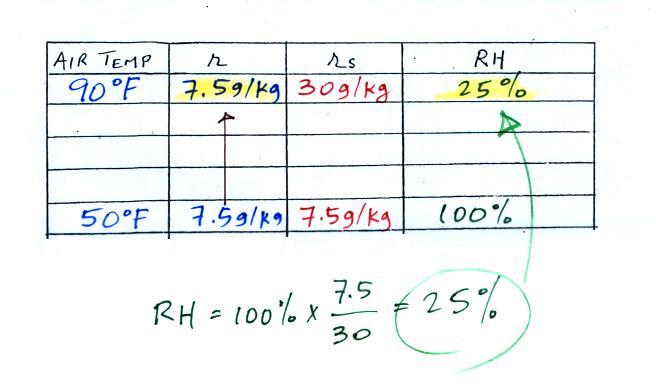
We do know that if we cool the 90 F air to 50 F the RH will become
100%. So on the 50 F row, we can set the mixing ratio equal
to the value of the saturation mixing ratio at 50 F, 7.5 g/kg.
Remember back to the three earlier examples. When
we cooled air to the the dew point, the mixing ratio didn't
change. So the mixing ratio must have been 7.5 all
along. Once we know the mixing ratio in the 90 F air
it is a simple matter to calculate the relative humidity, 25%.
The figure below is on p. 87 in the photocopied
ClassNotes. It
explains how you can dry moist air.
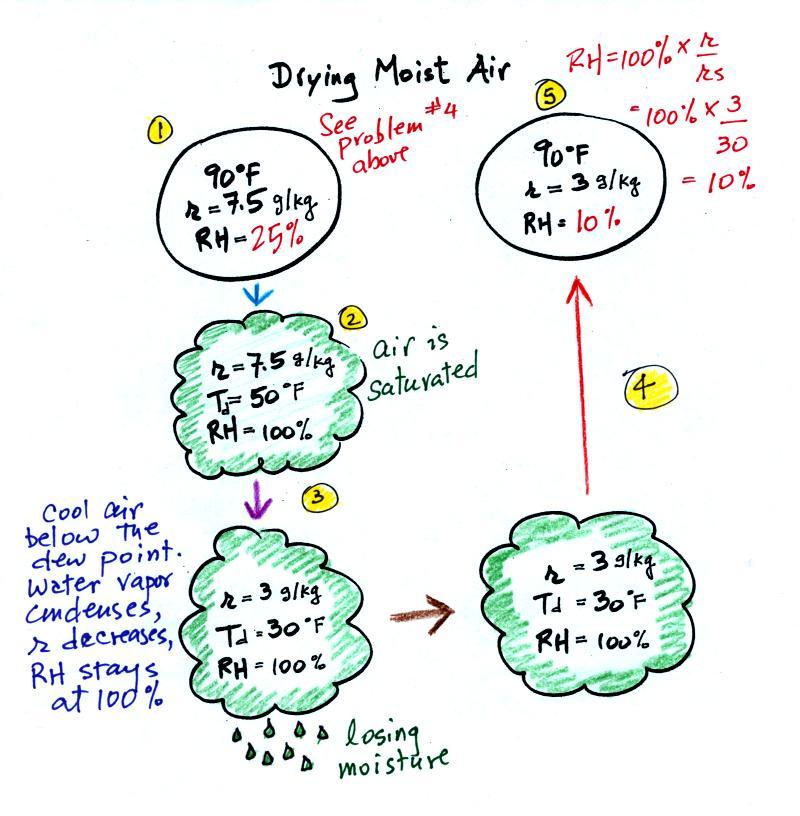
At Point 1 we start with some 90 F air with a relative humidity
of 25%, fairly dry air. These are the same numbers in
Example Problem #4 last Wednesday. We imagine cooling this
air to the dew point temperature, 50 F. While doing that the
mixing ratio, r, would stay constant. Relative humidity
would increase and eventually reach 100%. A cloud would form
(Pt. 2 in the figure above).
Then we continue to cool the air below the dew point, to 30
F. Air that is cooled below the dew point finds itself with
more water vapor than it can contain. The excess moisture
must condense (we will assume it falls out of the air as rain or
snow). Mixing ratio will decrease, the relative humidity
will remain 100%. When air reaches 30 F it contains 3 g/kg,
less than half the moisture that it originally did (7.5
g/kg). The air is being warmed back up to 90 F along Path
4. As it warms the mixing ratio remains constant. At
Point 5, the air now has a RH of only 10%.
Drying moist air is very similar to wringing moisture from a wet
sponge.
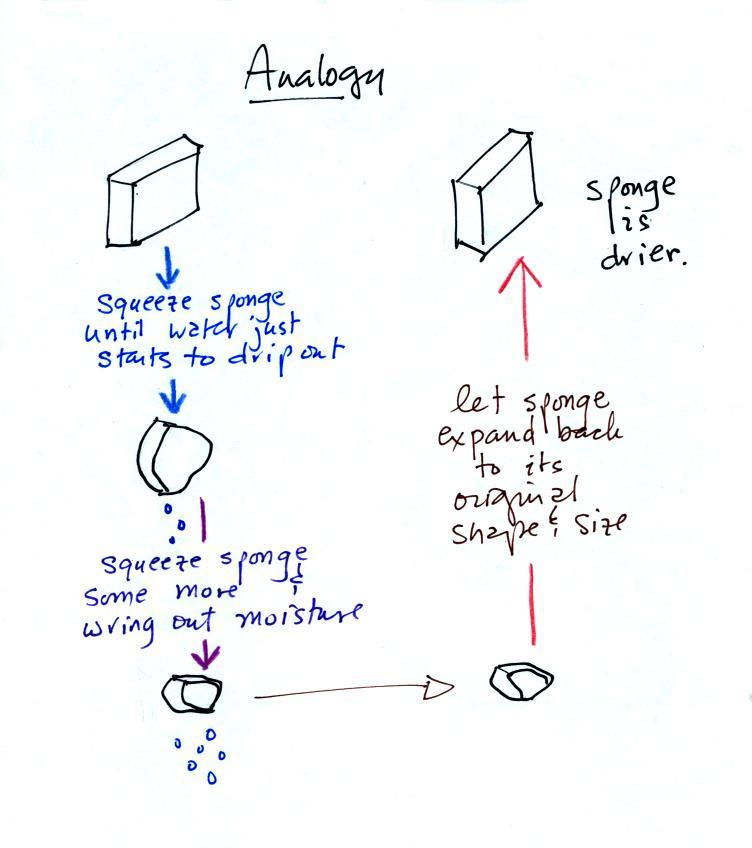
You start to squeeze the sponge and it gets
smaller. That's like cooling the air and reducing the
saturation mixing ratio, the air's capacity for water
vapor. At first squeezing the sponge doesn't cause
anything to happen (that's like cooling the air, the mixing
ratio stays constant as long as the air doesn't lose any water
vapor). Eventually water will start to drop from the
sponge (with air this is what happens when you reach the dew
point and continue to cool the air below the dew point).
Then you let go of the sponge and let it expand back to its
original shape and size (the air warms back to its original
temperature). The sponge (and the air) will be drier
than when you started.
This sort of process ("squeezing" water vapor out of moist air
by cooling the air below its dew point) happens all the
time. Here are a couple of examples (p. 87 again)
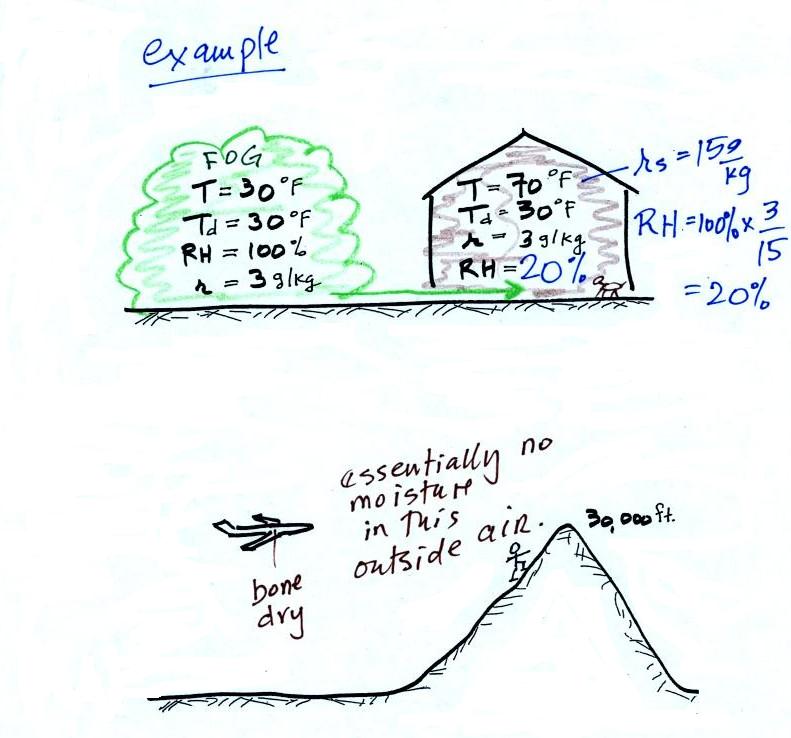
In the winter cold air is brought inside your house or
apartment and warmed. Imagine 30 F air with a RH of 100%
(this is a best case scenario, the cold air outdoors usually
has a lower dew point and is drier). Bringing the air inside
and warming it will cause the RH to drop from 100% to
20%.. Air indoors during the winter is often very
dry. This can cause chapped skin, can irritate nasal
passages, and causes cat's fur to become charged with static
electricity.
The air in an airplane comes from outside the plane. The
air outside the plane can be very cold (-60 F perhaps) and
contains very little water vapor (even if the -60 F air is
saturated it would contain essentially no water vapor).
When brought inside and warmed to a comfortable
temperature, the RH of the air in the plane will be
essentially 0%. Passengers often complain of dehydration
on long airplane flights. The plane's ventilation
system must add moisture to the air so that it doesn't get
that dry.
Next a much more important example of drying moist air (see
p. 88 in the photocopied ClassNotes).

We start with some moist but unsaturated air (the RH
is about 50%) at Point 1 (the air and dew point temperatures
would need to be equal in order for the air to be saturated). As it is
moving toward the right the air runs into a mountain and
starts to rise. Rising air expands and cools.
Unsaturated air cools 10 C for every kilometer of altitude
gain (this is known as the dry adiabatic lapse rate but isn't
something you need to remember). So after rising 1 km
the air will cool to 10 C which is the dew point.
The air becomes saturated at Point 2 (the air temperature and
the dew point are both 10 C). Would you be able to tell
if you were outdoors looking at the mountain? Yes, you
would see a cloud appear.
Now that the RH = 100%, the saturated air cools at a slower
rate than unsaturated air (condensation of water vapor
releases latent heat energy inside the rising volume of air,
this warming partly offsets the cooling caused by
expansion). We'll use a value of 6 C/km (an average
value). The air cools from 10 C to 4 C in next kilometer
up to the top of the mountain. Because the air is being
cooled below its dew point at Point 3, some of the water vapor
will condense and fall to the ground as rain. Moisture
is being removed from the air and the value of the mixing
ratio (and the dew point temperature) decreases.
At Point 4 the air starts back down the right side of the
mountain. Sinking air is compressed and warms. As
soon as the air starts to sink and warm, the relative humidity
drops below 100% and the cloud disappears. The sinking
unsaturated air will warm at the 10 C/km rate.
At Point 5 the air ends up warmer (24 C vs 20 C) and drier (Td
= 4 C vs Td = 10 C) than when it started out. The
downwind side of the mountain is referred to as a "rain
shadow" because rain is less likely there than on the upwind
side of the mountain. Rain is less likely because the
air is sinking and because the air on the downwind side is
drier than it was on the upslope side.
|
 |
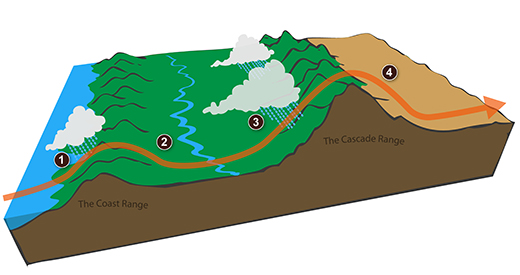
We can see the effects of a rainshadow illustrated well in
the state of Oregon. The figure above at left shows the
topography (here's the source
of that map). Winds generally blow from west to
east across the state.
Coming off the Pacific Ocean the winds first encounter a coastal
range of moutains. On the precipitation map above at right
(source)
you see a lot of greens and blue on the western sides of the
coastal range. These colors indicate yearly rainfall
totals that range from about 50 to more than 180 inches of rain
per year. temperate rainforests are found in some of these
coastal locations.
That's the Willamette River, I think, in between the coastal
range and the Cascades. This valley is somewhat drier than
the coast because air moving off the Pacific has lost some of
its moisture moving over the coastal range.
What moisture does remain in the air is removed as the winds
move up and over the taller Cascades. Yearly rainfall is
generally less than 20 inches per year on the eastern side, the
rain shadow side, of the Cascades. That's not too much
more than Tucson which averages about 12 inches of rain a year.
Most of the year the air that arrives in Arizona comes
from the west, from the Pacific Ocean (this changes in the
summer). It usually isn't very moist by the time it
reaches Arizona because it has travelled up and over the Sierra
Nevada mountains in California and the Sierra Madre mountains
further south in Mexico. The air loses much of its
moisture on the western slopes of those mountains.
A brief detour at this point to have a look at the grade
summary printouts. A color coded example is shown below
(values shown are class averages)
Doe_J
quiz1
-48 (170 pts possible) 71.8%
quiz2 -46 (165 pts possible) 72.1%
1.1
EC points (1.1 pts possible)
writing
scores: 0.0 (expt/book report) + 14.5 (1S1P pts
(average is 14.5))
writing percentage grade estimate: 86.4%
average (no quiz scores
dropped): 74.8% + 1.1 = 75.9%
average (lowest quiz score dropped): 75.7% + 1.1
= 76.8%
Your grades on the two quizzes are shown first in dark green (I didn't record your
score on the Practice Quiz and it isn't shown). There are
two more quizzes this semester (Quiz #3 is Thursday next week,
Nov. 7).
Next in dark brown are the
number of extra credit points you have earned from turning in
Optional Assignments. It is possible to have earned 1.1
pts at this point, a handful of students have. By
the end of the semester you will have had an opportunity to have
earned at least 3 pts of extra credit (perhaps a little more
than that).
Your score on either an Expt. #1, Expt. #2 or a book report is
shown next in purple.
Many students haven't yet turned in a report. They'll find
a 0 listed here and a short message at the bottom of their grade
summary saying that an average score was used by the computer to
provide a reasonable estimate of their writing grade. The
report score is followed by the total number of 1S1P points you
have earned (the class average is 14.5 which is a bit low but
still on target to earn 45 pts by the end of the
semester). The report points and the 1S1P points are added
and a writing percentage grade is computed. The computer
has taken into account the fact that you can't have earned 45
1S1P points at this point in the semester. By the end of
the semester if you have a decent experiment report score and 45
1S1P pts the writing percentage grade should be close to, maybe
a little over, 100%. It is important to understand
that the writing grade shown on your grade summary is not
"locked in." If you stop writing 1S1P reports your writing
grade will drop by the end of the semester. So keep
writing 1S1P reports until you have reached the 45 pt maximum
number of 1S1P pts allowed.
Finally the quiz scores and the writing percentage grade are
themselves averaged, the extra credit is added on and your
overall grade is shown in this reddish
color. No quiz scores have been dropped in
the first average. This is the average that has to be
90.0% or above on the last day of classes in order to get out of
the Final Exam. If you do have to take the Final Exam, the
second average (with your lowest quiz score dropped) will be
used together with your Final Exam score to determine your
overall grade.
Another important point to keep in mind. The grade
estimate attempts to determine what you will end up with at the
end of the semester if you keep doing like you have done up
to this point. With two quizzes left and lots of
writing still to do there is time for significant
improvement. It is also possible for your grade to drop
between now and the end of classes if you stop performing as you
have been.
I'll try to handout
another grade summary following Quiz #3 and a 3rd grade summary
for sure after Quiz #4 so students will know whether they need
to take the Final Exam or not.
NOTE: Please check to
be sure the grades listed on your summary are correct. And,
as far as graded work is concerned, we're at about the halfway
point. There is still time to earn a lot of 1S1P points and
there are two quizzes left to take. So your grade can change
significantly between now and the last day of classes.
Next in our sequence of topics was
measuring humidity. One of the ways of measuring humidity is
to use a sling (swing might be more descriptive) psychrometer.
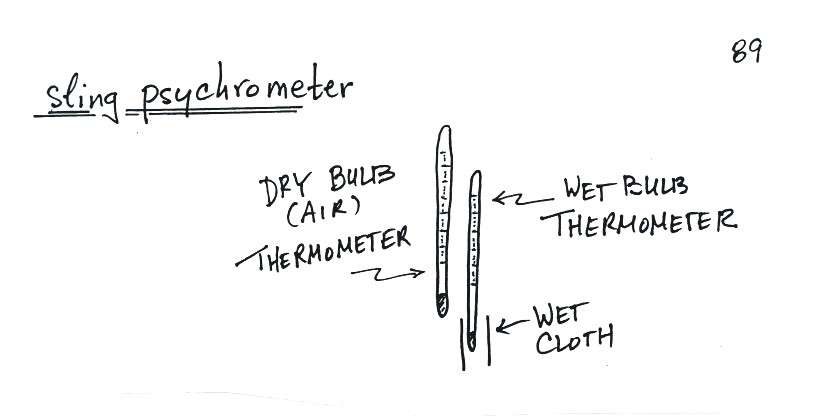
A sling psychrometer consists of two
thermometers mounted side by side. One is an ordinary
thermometer, the other is covered with a wet piece of
cloth. To make a humidity measurement you swing the
psychrometer around for a minute or two and then read the
temperatures from the two thermometers. The difference
between the dry and wet bulb temperatures can be used to
determine relative humidity and dew point (you look up RH and
Td in a table, it's not something you can easily
calculate).
I'm sorry about my tendency to beat some concepts to death,
please bear with me. But this is a pretty good situation
of where you can take some basic concepts and use and apply
them to really understand a concept thoroughly.
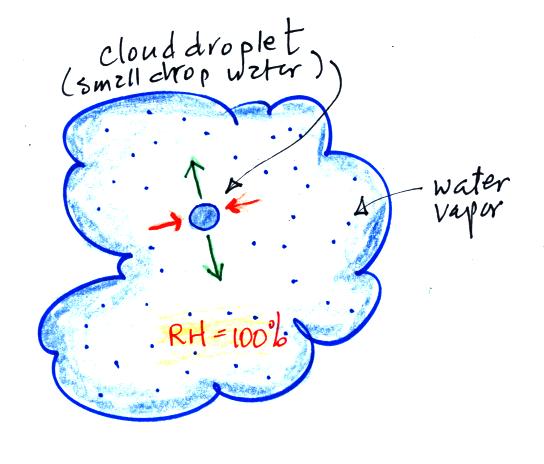
One concept that will use again is the fact that when
water (a drop of water, water in a glass, etc) is
surrounded by saturated air (RH = 100%) any evaporation
will be balanced by an equal amount of condensation.
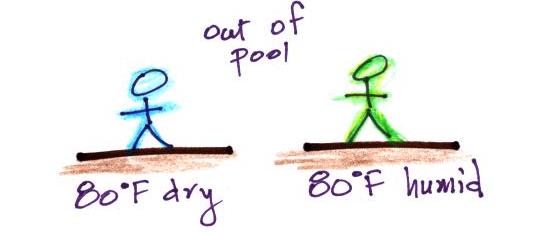
If you step out of a pool on a foggy day you wouldn't dry
off. Water would evaporate from your body but it
would be matched with an equal amount of condensation.
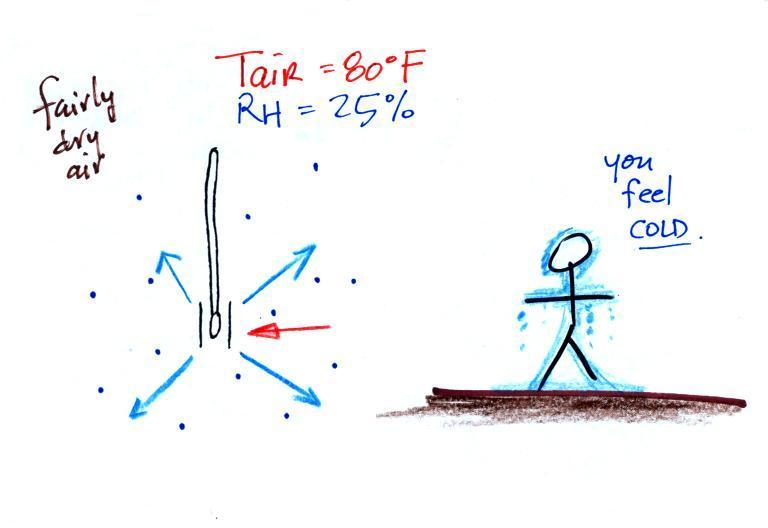
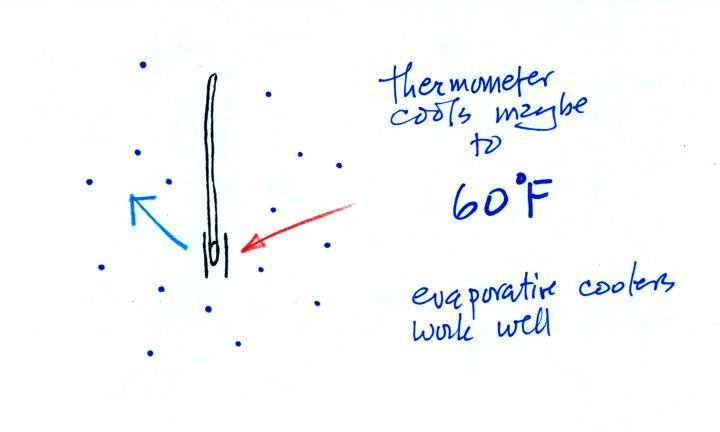
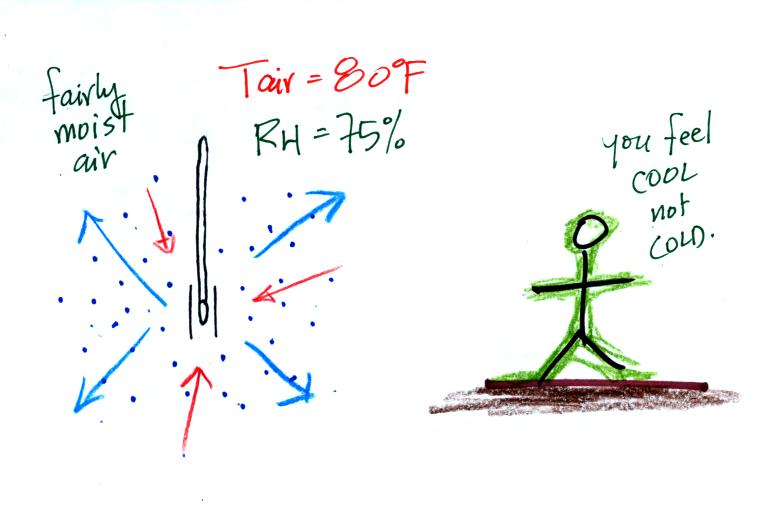
The figure shows
what will happen as you start to swing the wet bulb
thermometer. Water will begin to evaporate from the
wet piece of cloth. The
amount or rate of evaporation will depend on the
water temperature (the 80 F value
was just made up in this example). Warm water
evaporates at a higher rate than cool water (think of a
steaming cup of hot tea and a glass of ice tea).
The evaporation is shown as blue arrows because this will
cool the thermometer. The same thing would happen if
you were to step out of a swimming pool on a warm dry day,
you would feel cold. Swamp coolers would work well
(too well sometimes) on a day like this.
The figure at upper left also shows one arrow of
condensation. The amount or rate of
condensation depends on how much water vapor is in the
air surrounding the thermometer. In
this case (low relative humidity) there isn't much water
vapor. The condensation arrow is orange because the
condensation will release latent heat and warm the
thermometer.
Because there
is more evaporation (4 arrows) than condensation (1
arrow) the wet bulb thermometer will drop.
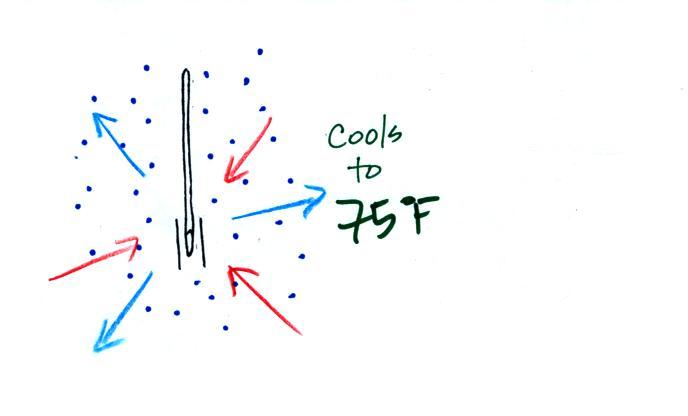
The wet thermometer will cool but there's a
limit to how cold it will get. We imagine that the
wet bulb thermometer has cooled to 60 F. Because the
wet piece of cloth is cooler, the rate of evaporation has
decreased. The wet bulb thermometer has cooled to a
temperature where the evaporation and condensation are in
balance. The thermometer won't cool any further.
You would measure a
large difference (20 F) between the dry and wet bulb
thermometers on a day like this when the air is relatively
dry.
Here's the situation on a
moister day. There's enough moisture in the air
to provide 3 arrows of condensation. You wouldn't feel as cold if you stepped out of a
pool on a warm humid day like this. Swamp coolers
wouldn't provide much cooling on a humid day like this.
The wet thermometer only cools a little bit
before the rates of evaporation and condensation are
equal.
Here's a summary
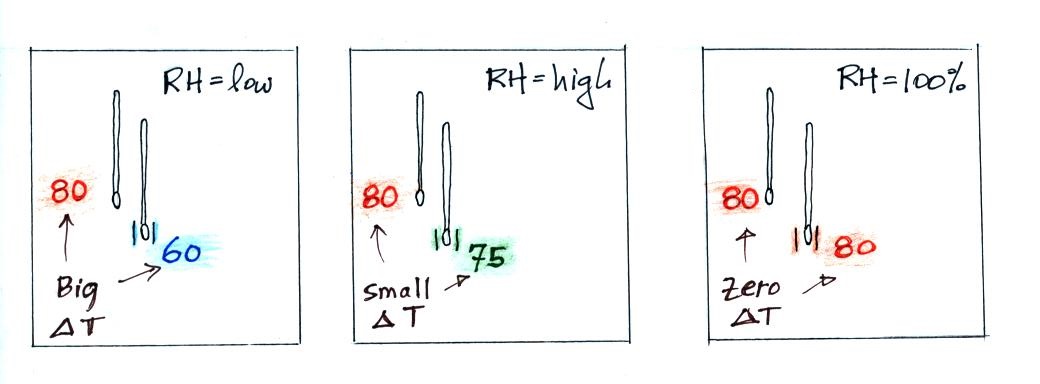
A
large difference between the dry and wet bulb
temperatures means the relative humidity is
low.
A small difference means
the RH is higher.
No difference means
the relative humidity is 100%.
Evaporative cooling will
make you feel cold if you get out of a swimming pool on a
warm dry day. You won't feel as cold if
the air is humid and the relative humidity is high.
This might remind you of something similar that we covered
earlier in the semester.
If you said "wind chill" you're right.
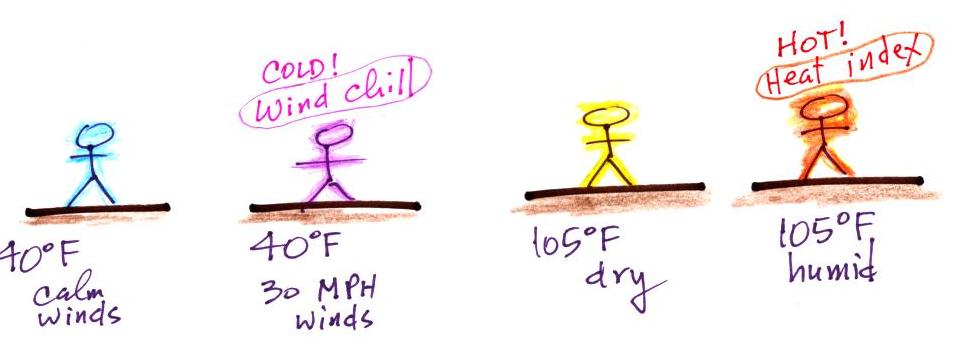
We learned that a 40 F day with 30 MPH
winds will feel colder (because of increased transport of
energy away from your body by convection) than a 40 F day with
no wind. The wind
chill temperature tells you how much colder it will feel
( a thermometer would measure the same temperature on both the
calm and the windy day). If your body isn't able to keep
up with the heat loss, you can get hypothermia
and die.
There's something like that involving heat
and humidity. Your body tries to stay cool by
perspiring. You would feel hot on a dry 105 F day.
You'll feel even hotter on a 105 F day with high relative
humidity because your sweat won't
evaporate as quickly. The heat index
measures how much hotter you'd feel. The combination of heat
and high humidity is a serious, potentially deadly, weather
hazard because it can cause heatstroke
(hyperthermia).
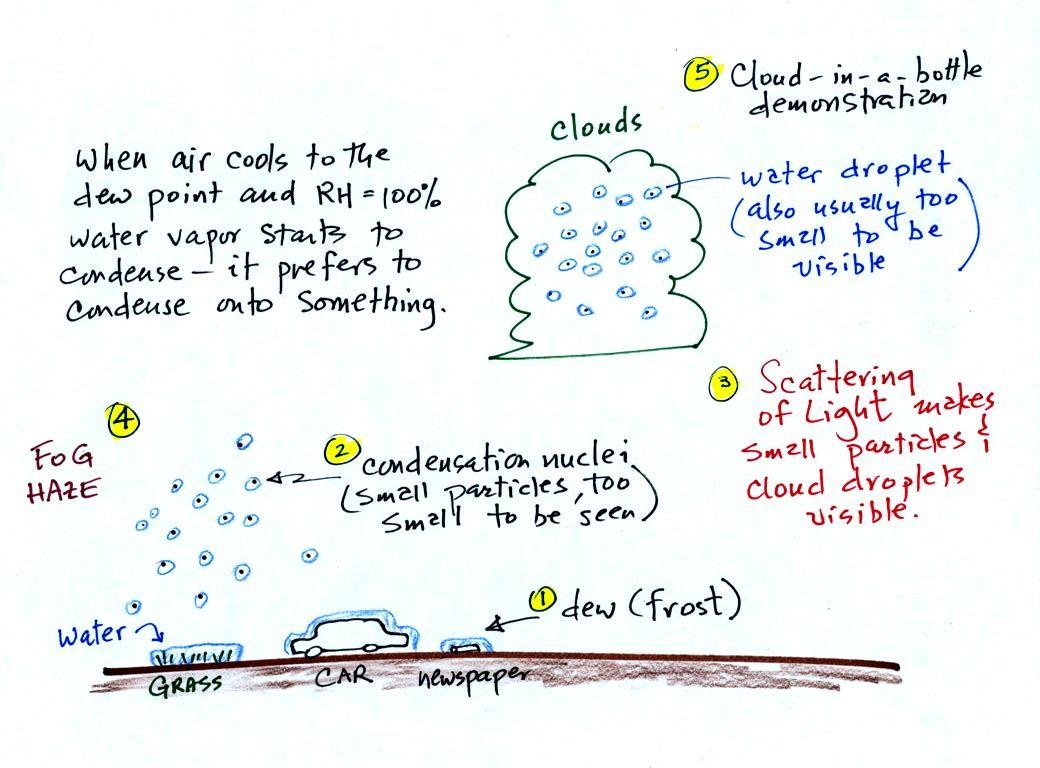
A variety of things can happen when you
cool air to the dew point and the relative humidity increases
to 100%. Point 1 shows that when moist air
next to the ground is cooled to and below the dew point, water
vapor condenses onto (or is deposited onto) the ground or
objects on the ground. This forms dew, frozen dew, and
frost.
Air above the ground can also be cooled to the dew
point. When that happens (Point 2 above) it is much
easier for water vapor to condense onto something rather than
just forming a small droplet of pure water. In
air above the ground water vapor condenses onto small
particles in the air called condensation nuclei. Both
the condensation nuclei and the small water droplets that form
on them are usually too small to be seen with the naked
eye. We can tell they are present (Point 3) because they
scatter sunlight and make the sky hazy. As humidity
increases dry haze turns to wet haze and eventually to fog
(Point 4). When air well above the ground, clouds
can form.
On Thursday we'll come back to the role condensation nuclei
play in cloud formation and create a cloud in a bottle.
Then we'll look at identifying and naming clouds.