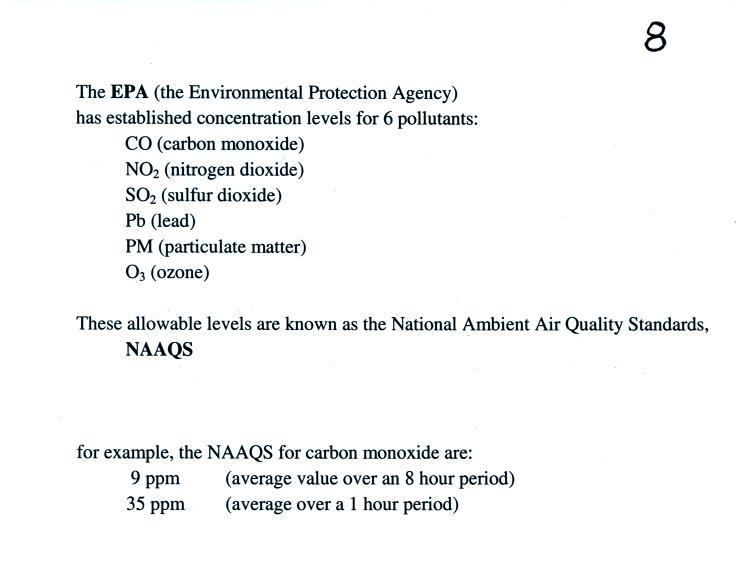
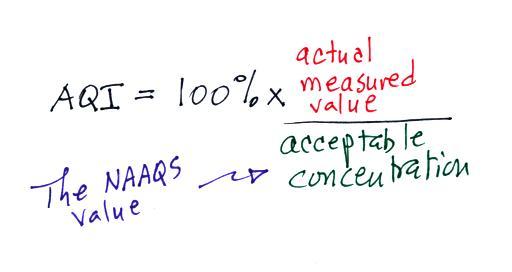
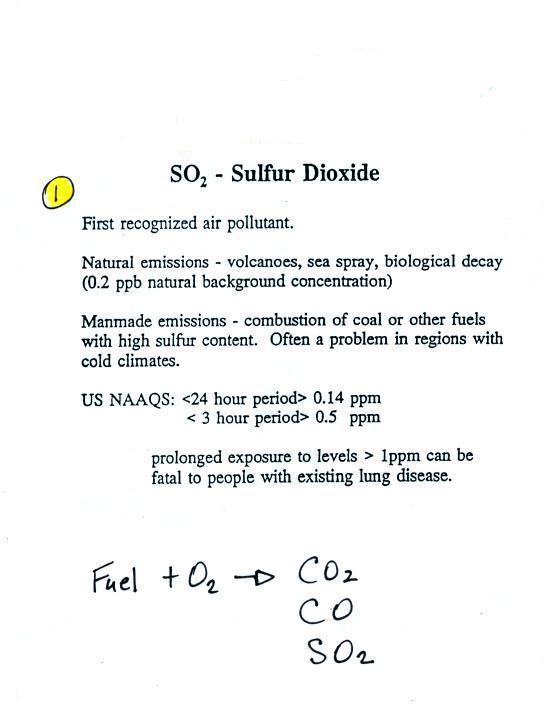
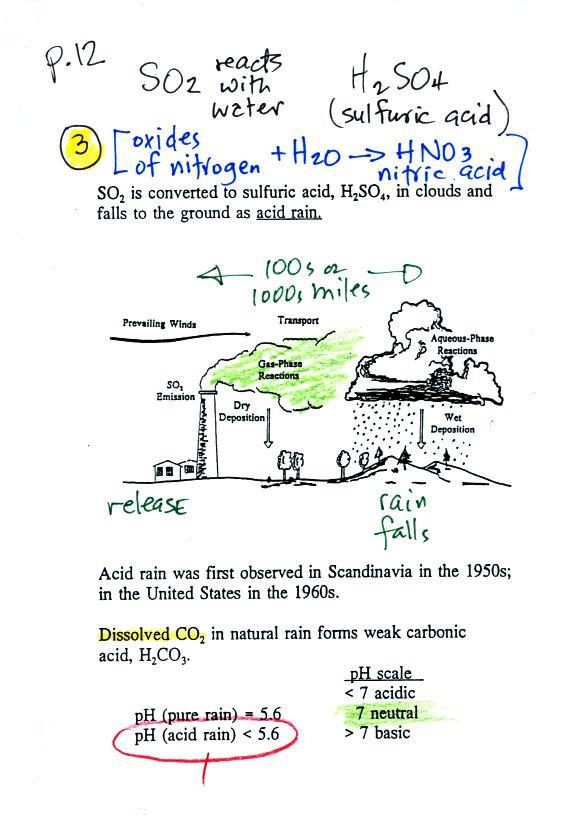
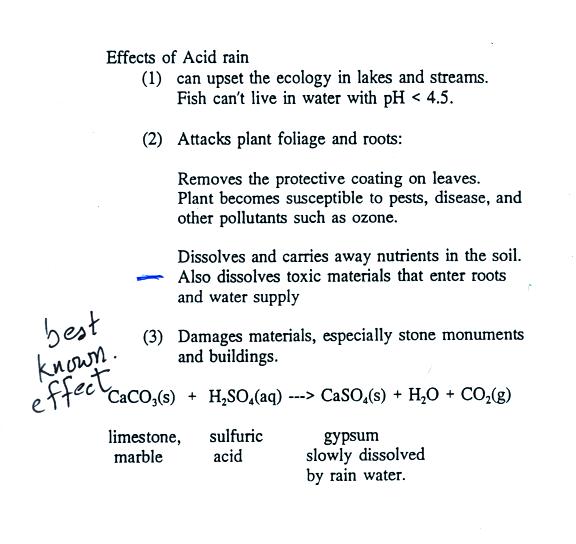
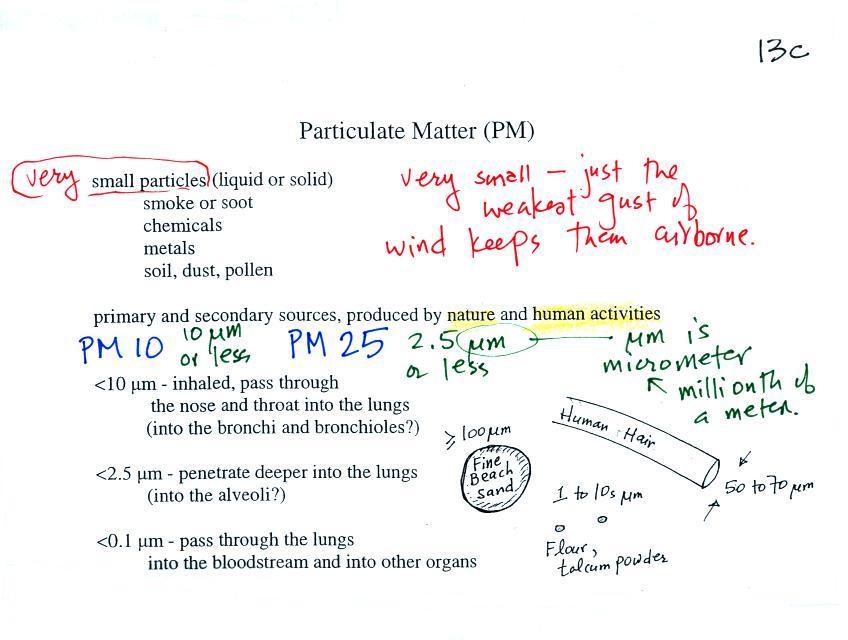
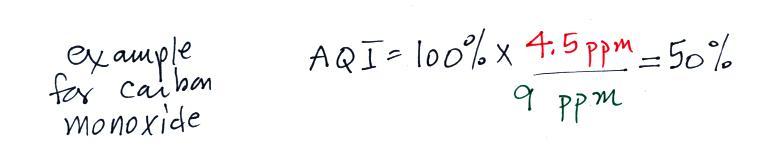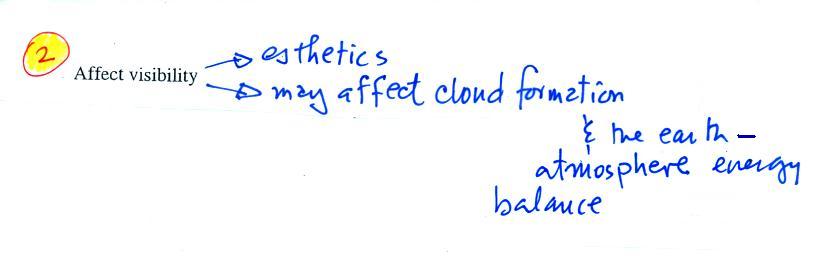Particulate matter can be
produced naturally (wind blown dust, clouds above volcanic
eruptions, smoke from lightning-caused forest and brush
fires). Human activities also produce
particulates. Gases sometimes react in the atmosphere
to make small drops or particles (this is what happened in
the photochemical smog demonstration). Just the
smallest, weakest gust of wind is enough to keep particles
this small suspended in the atmosphere.
One of the main concerns
with particulate pollution is that the small particles might
be a health hazard ( a health advisory is sometimes issued
during windy and dusty conditions in Tucson)
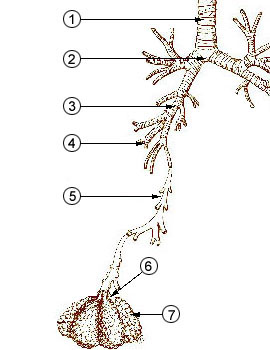
Particles with dimensions of 10 µm
and less can be inhaled into the lungs (larger particles get
caught in the nasal passages). These inhaled particles
may be poisonous, might cause cancer, damage lung tissue, or
aggravate existing respiratory diseases. The smallest
particles can pass through the lungs and get into the blood
stream (just as oxygen does) and damage other organs in the
body.
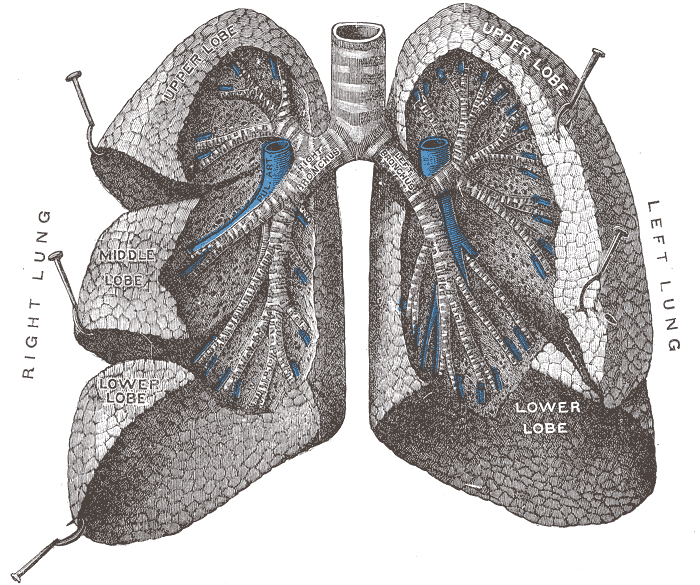
The figure below identifies some of the parts of the
human lung mentioned above.