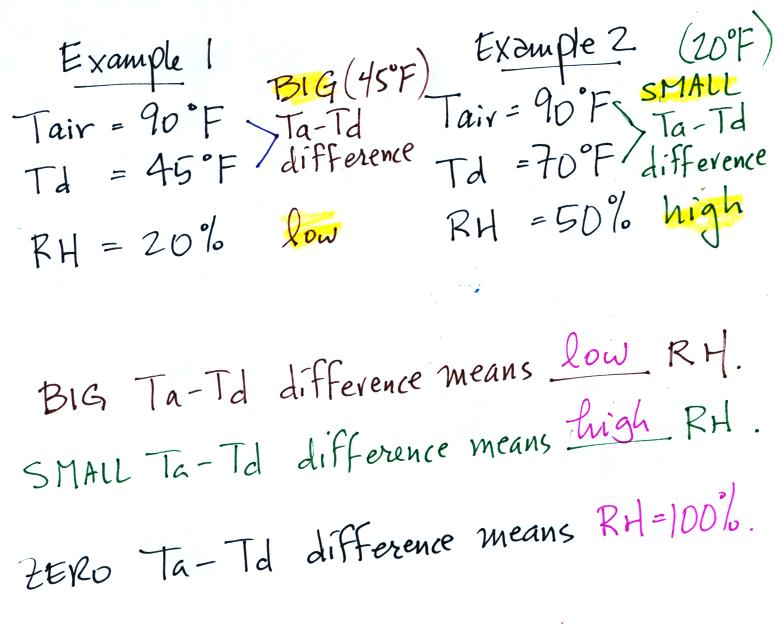
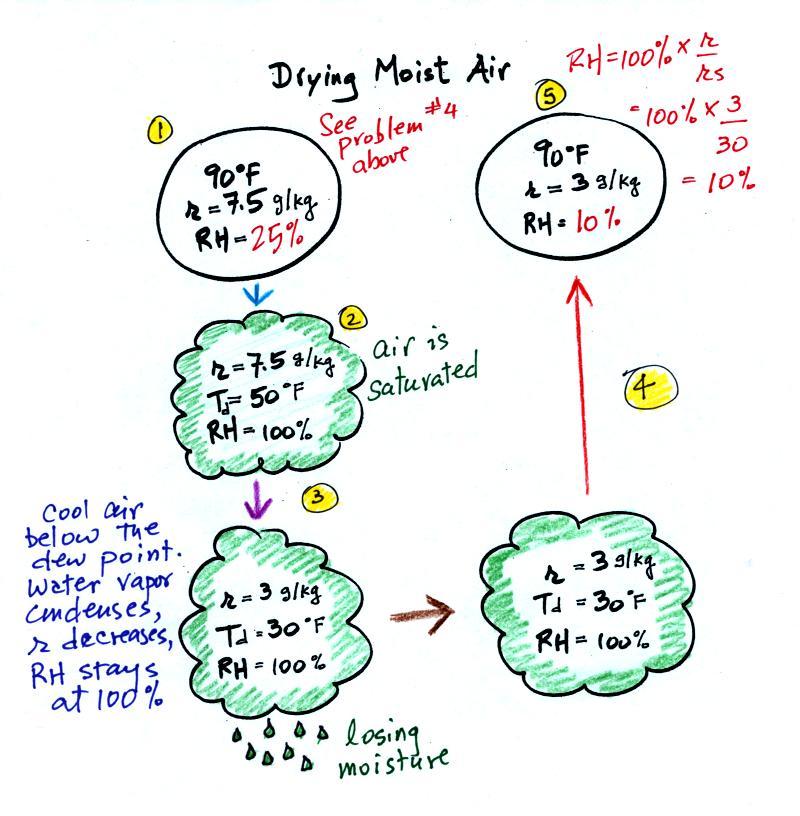
| Tair = 90 F |
RH = 20% |
| r = 6 g/kg |
Td = 45 F |
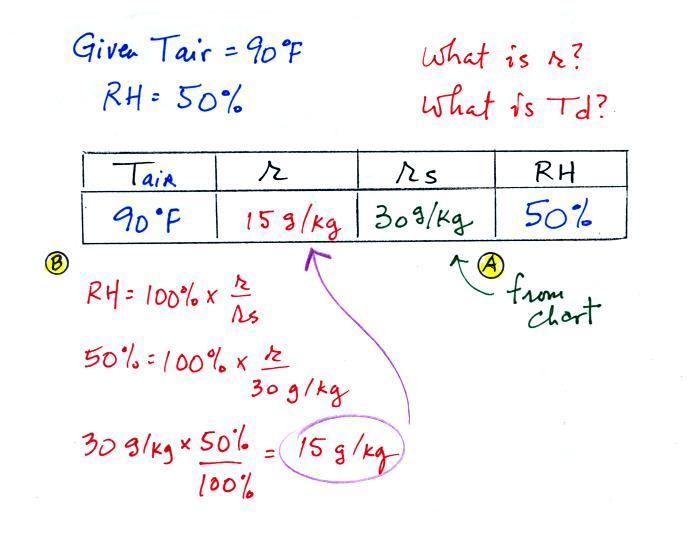
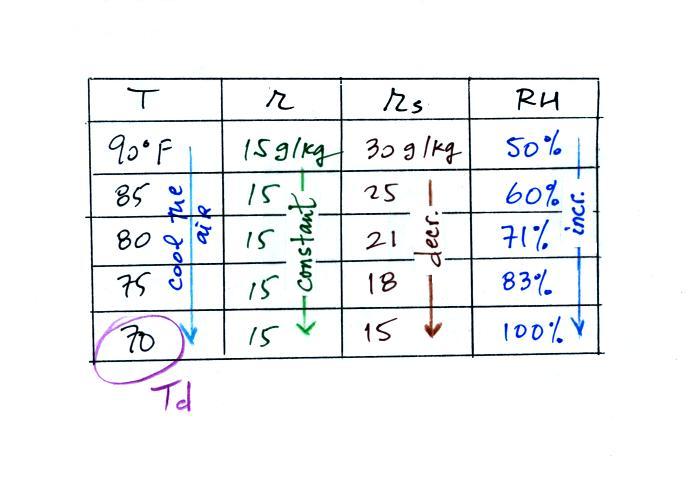
| Tair = 90 F |
RH = 50% |
| r = ? |
Td = ? |

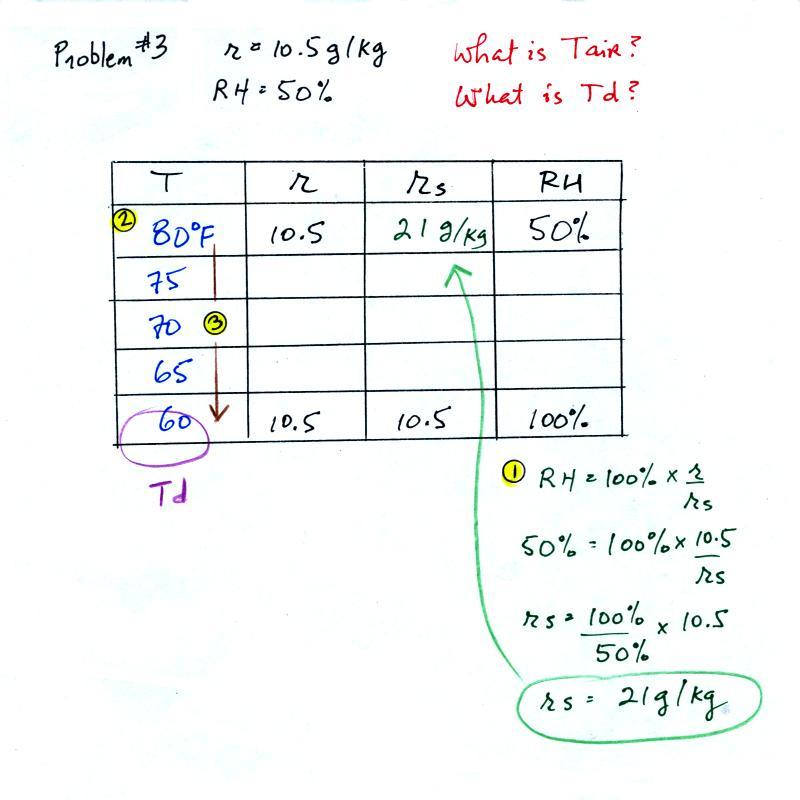
| Tair = ? |
RH = 50% |
| r = 10.5 g/kg |
Td = ? |
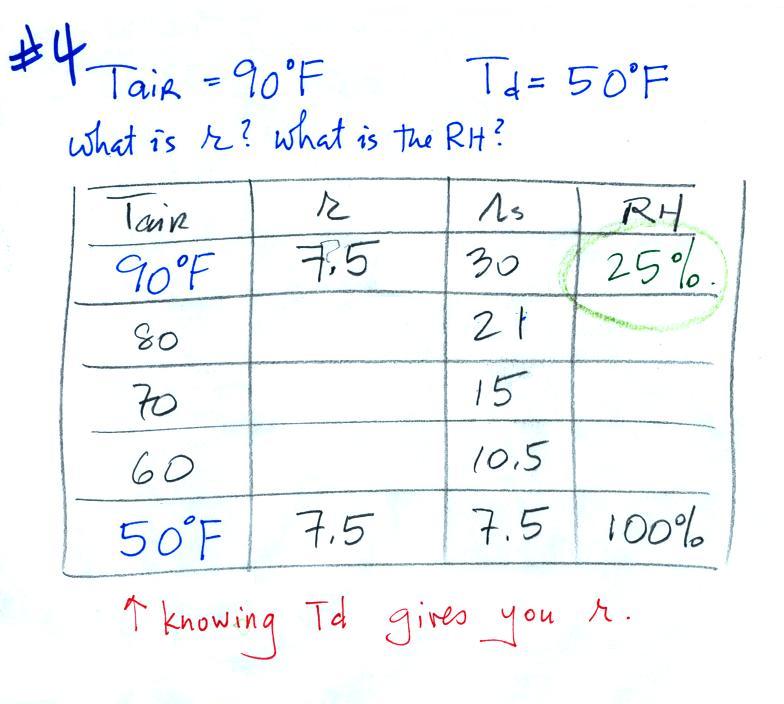
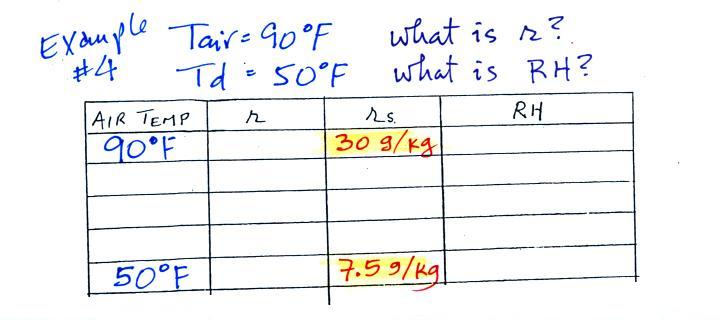
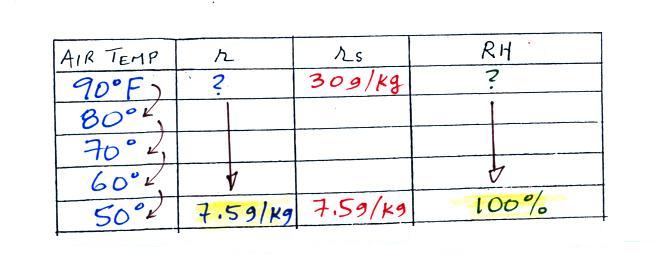
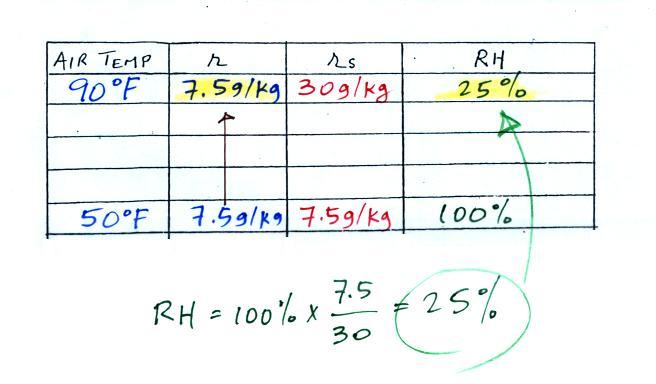
| Tair = 90 F |
RH = ? |
| r = ? |
Td = 50 F |

 |
 |
 |
 |
 |
 |
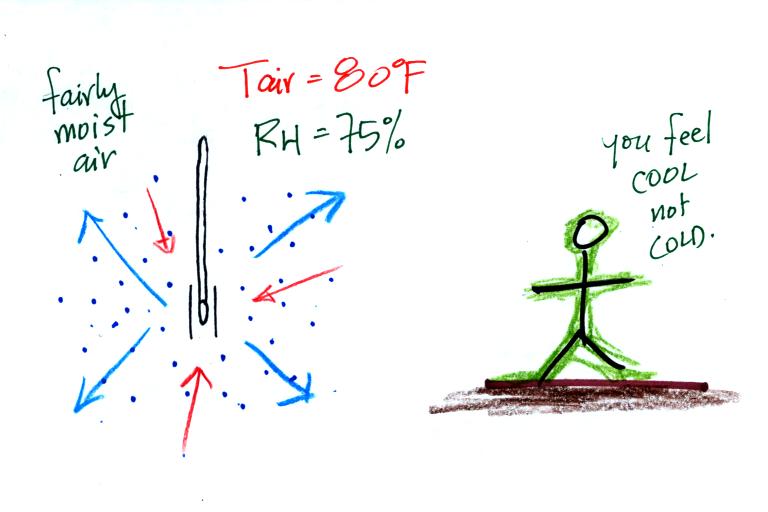
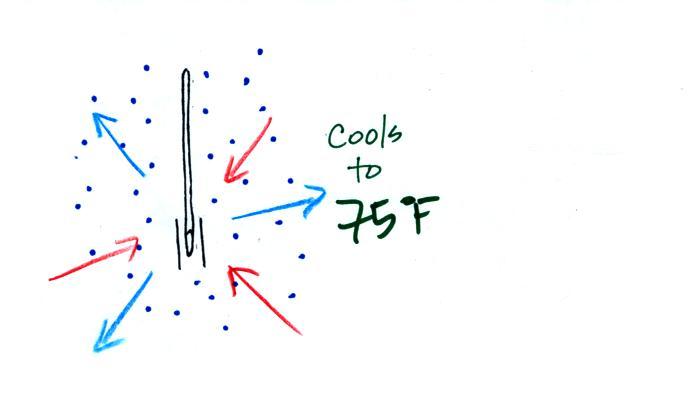
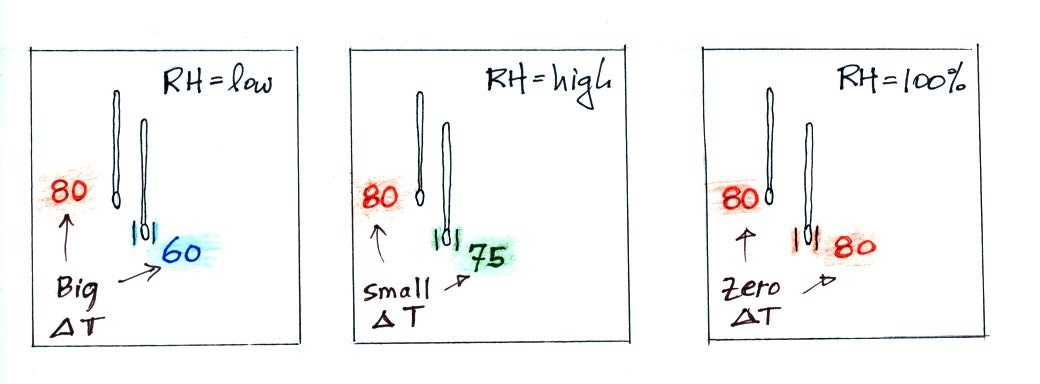
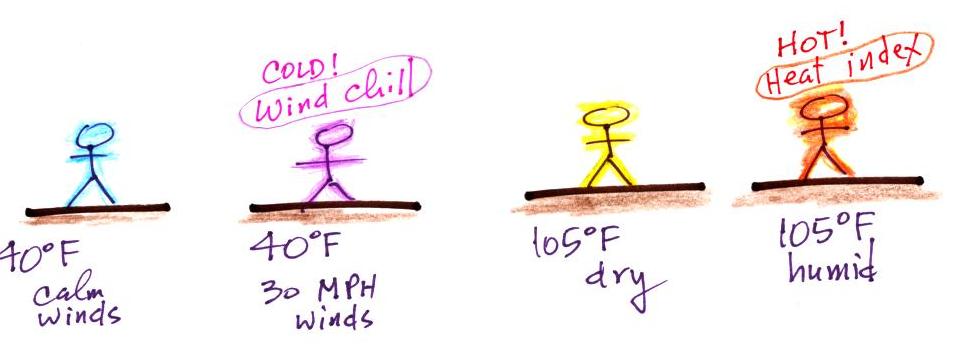
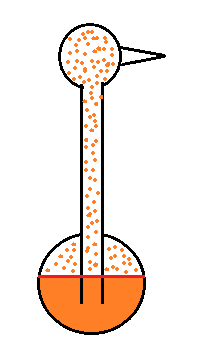
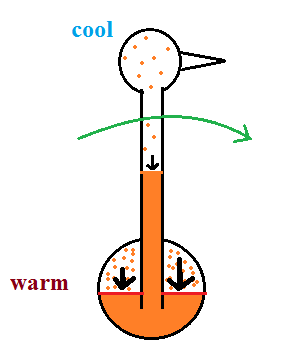
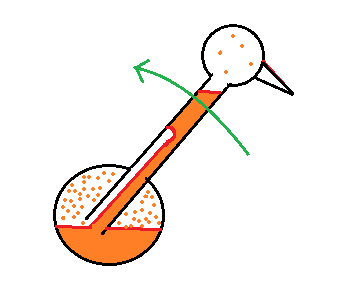

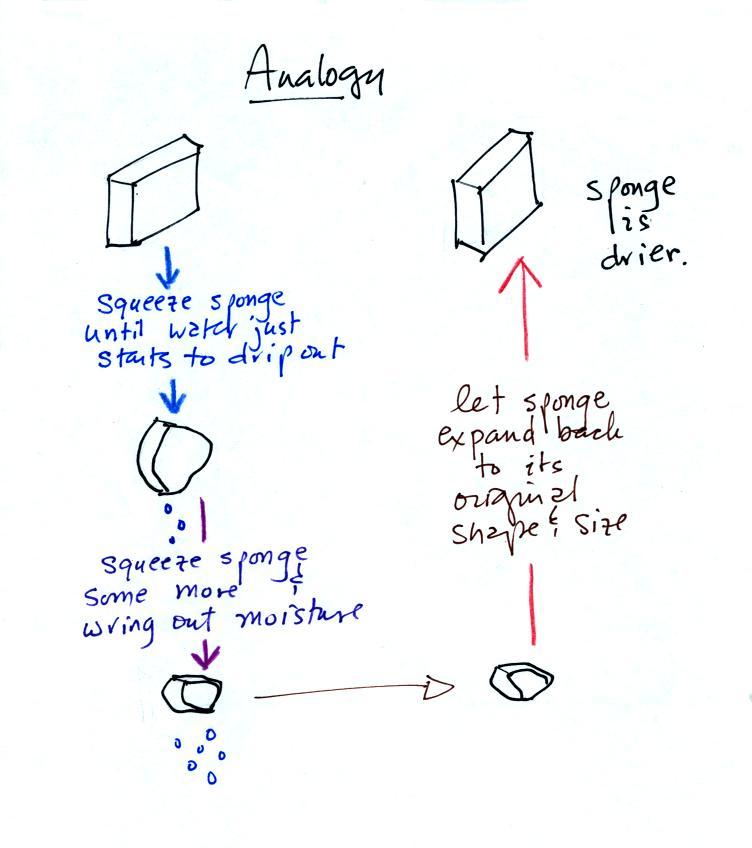
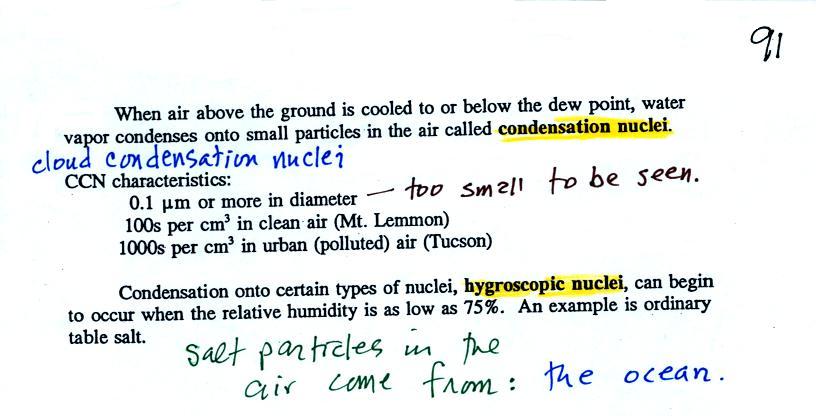
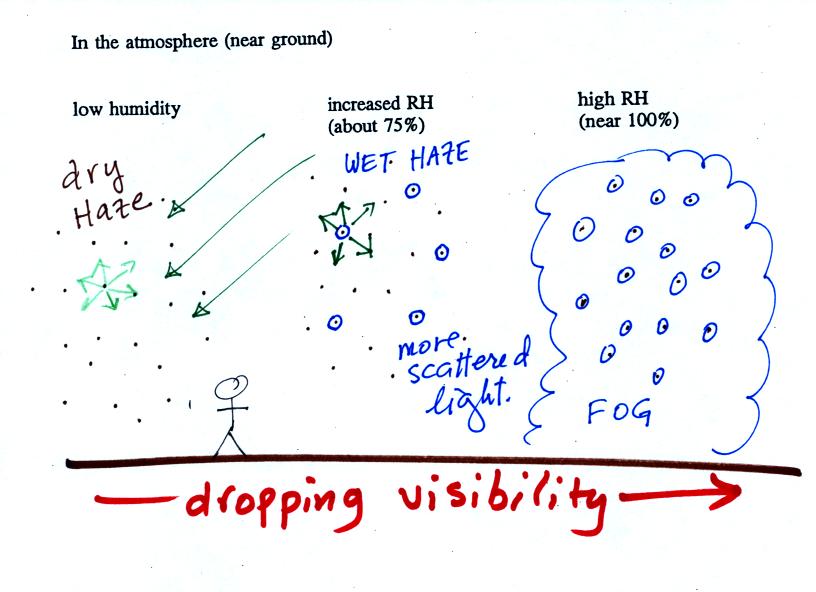
| it is much
easier for water vapor to condense onto small particles called condensation nuclei |
it is much harder for water
vapor to just condense and form small droplets of pure water |


 |
 |
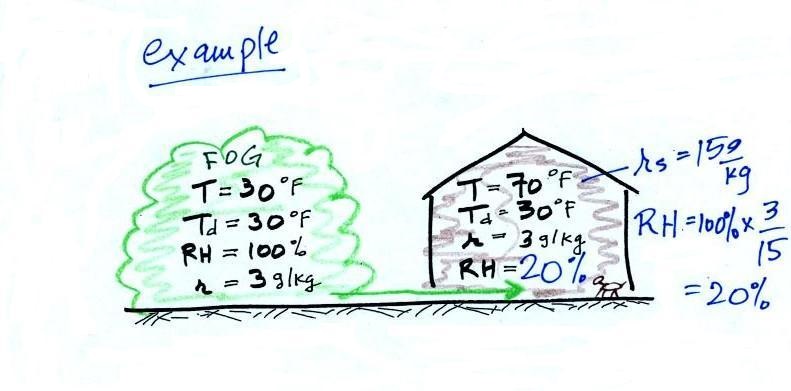
| Thin fog
(perhaps even wet haze) with pretty good visibility (source of the image) |
Thick fog (visibility was less than 500 feet) (source of the image) |