

 |
 |
 |
| from: http://www.webelements.com/oxygen/ The web elements site credits Prof. James Marshall's Walking Tour of the Elements. |
from: http://en.wikipedia.org/wiki/Oxygen Wikipedia credits U.S. Air Force Staff Sgt. Jim Araos. |
A nice picture of liquid oxygen's pale blue
color from
this source. |


| The noble gases have full valence electron shells. Valence electrons are the outermost electrons of an atom and are normally the only electrons that participate in chemical bonding. Atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons (take electrons from or give electrons to atoms of different materials). |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
| The "diving lady" sign was recently
restored. photo credit: Michael McKisson |
A sign at the Hotel Congress photo credit: David Sanders, Arizona Daily Star |
King Fisher restaurant photo credit: David Sanders, Arizona Daily Star |
Rialto Theater in downtown Tucson photo credit: Rich Facun, Arizona Daily Star |




 |
 |
 |
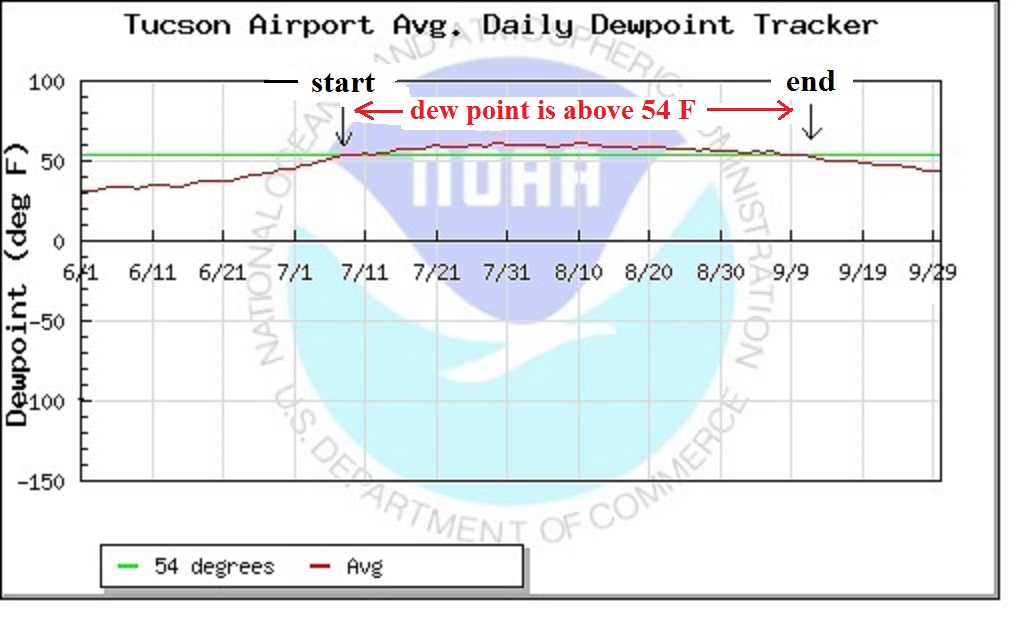
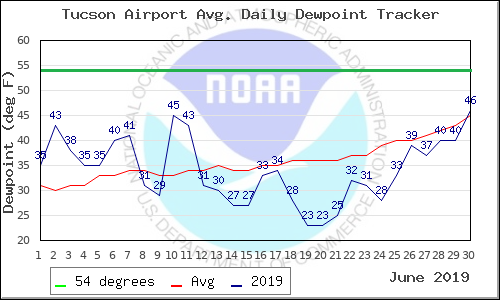
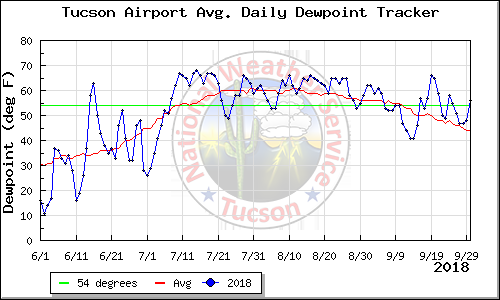
data for September 2019 will be
added later in the
semester
|
 |
 |
 |
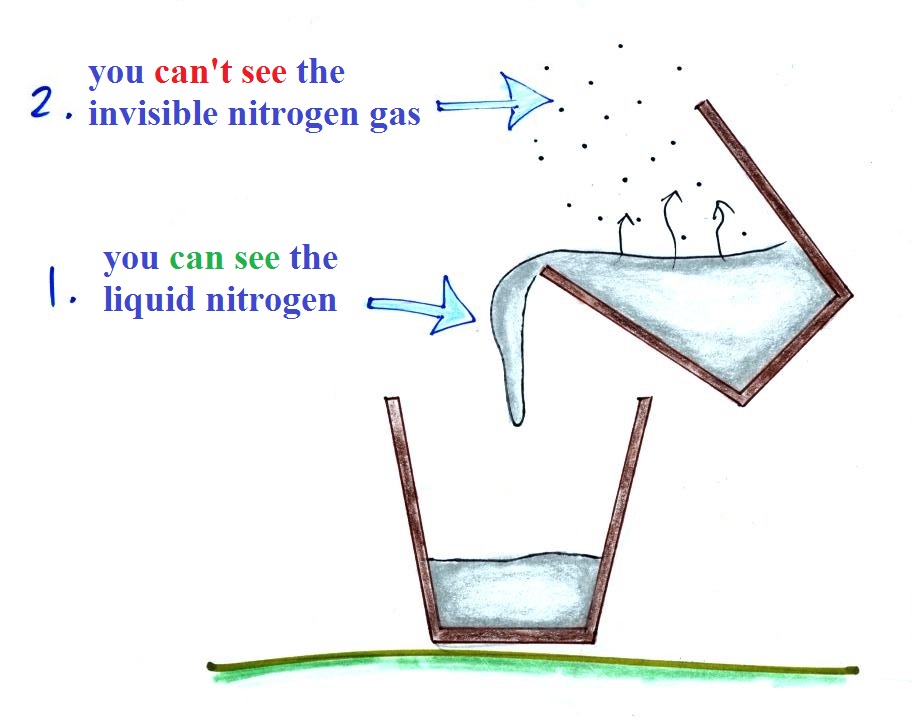
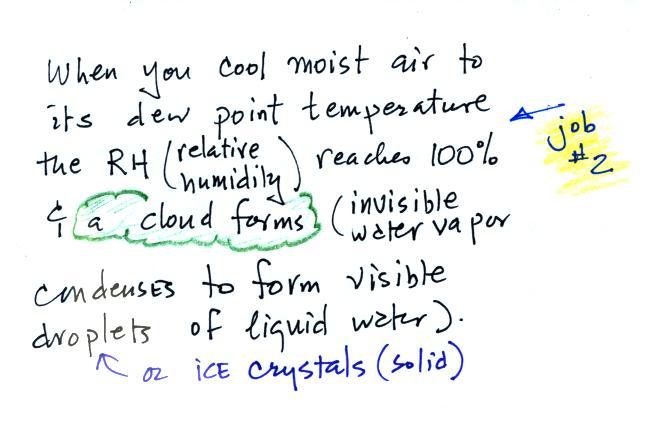
 The cloud
that you can see is water vapor that has
condensed to form small drops of water
or crystals of ice.
|

 |
 |
 |
|
| Bromine in
both liquid and gaseous phases. Bromine and
mercury are the only two elements that exist as
liquids at room temperature. The bromine
is in a sealed glass ampoule inside an acrylic
cube. Bromine could be safely brought to
class in a container like this. I
actually have a sample of mercury in a cube like
this and will bring it to class during the
semester. Here's what Webelements.com says about bromine: " It is a serious health hazard, and maximum safety precautions should be taken when handling it." I'm not sure what maximum safety precautions are, so I probably shouldn't be bringing it to class. This photo was taken by Alchemist-hp and was Picture of the Day on the English Wikipedia on Oct. 29, 2010. |
Chlorine (Cl2) I found this image here |
Iodine Also an element that is normally found in solid form. The solid sublimes, i.e. it changes directly from solid to gas (you would probably need to heat the solid iodine to produce gas as deeply colored as seen in the picture above). source of this image I think we can probably handle iodine safely and might well bring some to class. |
Nitrogen dioxide (NO2) An important pollutant. I used to make this in class but I've read that you can inhale a fatal dose of NO2 before showing any symptoms. NO2 also has an anesthetic effect - it can deadens your sense of smell. |



