Monday, October 7
We'll review and finish latent heat energy transport
page 58. Then we'll
move into the first part of the section on electromagnetic
radiation - page 59, page 60, page 61 and page 62.
Latent heat energy transport -- Examples of how energy
transport takes place.
Two more figures to
illustrate how latent heat energy transport can carry
energy from location to another. This first one is
my favorite, it ties everything together.
1. You've just stepped out of the shower and are covered
with water. The water is evaporating and energy is being
taken from your body.
2. The water vapor (containing the energy taken from
your body), drifts into the kitchen where it finds a cold can
sitting on a table.
3. Water vapor comes into contact with the cold can and
condenses. The hidden latent heat energy in the water
vapor is released into the can and warms the drink
inside.
Without you even
leaving the bathroom,
energy has effectively been transported from your warm
body to the cold can in the kitchen.
Here's what happens on a much grander scale in the
atmosphere.

We start in this picture in the tropics where there is often a
surplus of sunlight energy. Some of the incoming sunlight
evaporates ocean water. The resulting water vapor moves
somewhere else and carries hidden latent heat energy with it. This
hidden energy reappears when something (air running into a
mountain and rising, expanding, and cooling) causes the water
vapor to condense. The condensation releases energy into the
surrounding atmosphere. This would warm the air.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in a place like Tucson.
Now we get started on the 4th and
most important energy transport, electromagnetic radiation
Energy transport by electromagnetic
radiation
It's time to tackle electromagnetic (EM)
radiation, the 4th and most important of the energy transport
processes (it's the most important because it can transport
energy through empty space (outer space)).
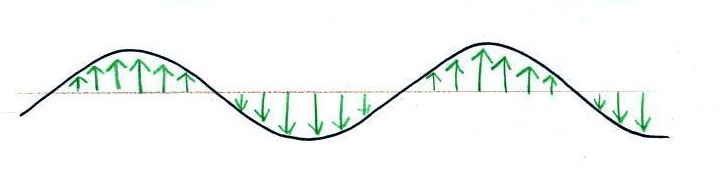
Many introductory textbooks depict EM
radiation with a wavy line like shown above. They don't
usually explain what the wavy line represents.
The wavy line just connects the tips of a bunch of "electric
field arrows". But what exactly are electric field arrows?
An electric
field arrow (vector)
just shows the direction
and
gives you an idea of the strength
of the electrical force
that would be exerted on
a positive charge at
that position.
It's just like an arrow painted on a street showing you what
direction to drive.
Electromagnetic (EM) radiation
Now we'll use what we know about electric field arrows (electric
field for short) to start to understand electromagnetic
radiation. How is it able to carry energy from
one place to another. You'll find most of the following on
p. 60 in the photocopied ClassNotes.
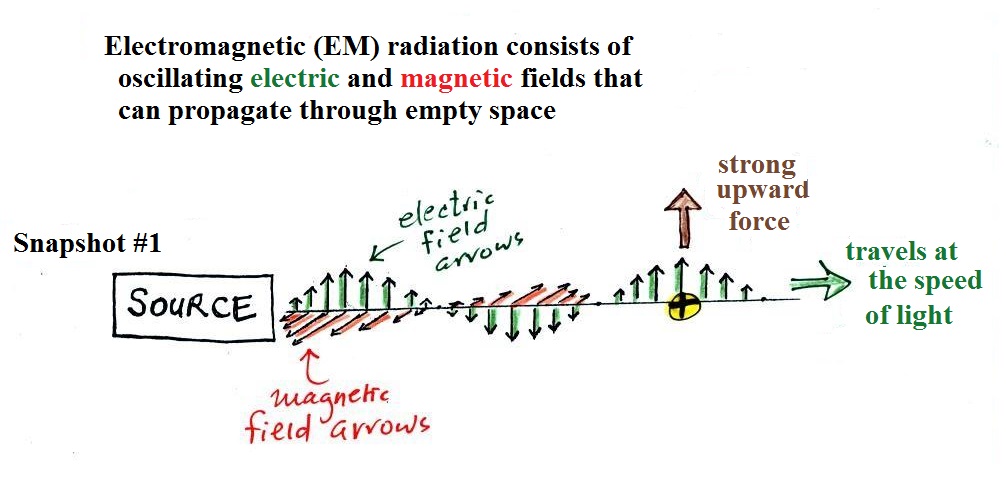
We imagine turning on a source of EM radiation and then a
very short time later we take a snapshot. In that time the
EM radiation has traveled to the right (at the speed of
light). The EM radiation is a wavy pattern of electric and
magnetic field arrows. We'll ignore the
magnetic field arrows. The E field arrows sometimes point
up, sometimes down. The pattern of electric field arrows
repeats itself.
Note the + charge near
the right side of the picture. At the time this picture
was taken the electric field at the position of the + charge points upward.
There is a fairly strong upward pointing force being exerted on
the + charge.
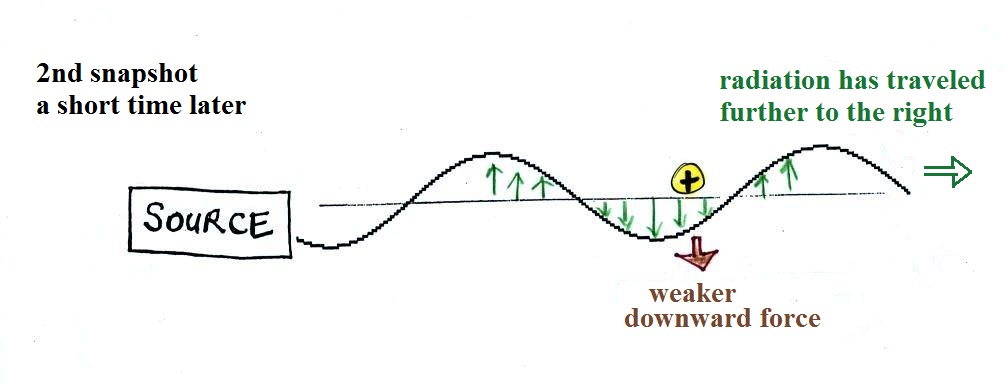
This picture above was taken a short time after the first
snapshot after the radiation had traveled a little further to
the right. The EM radiation now exerts a somewhat weaker
downward force on the +
charge.
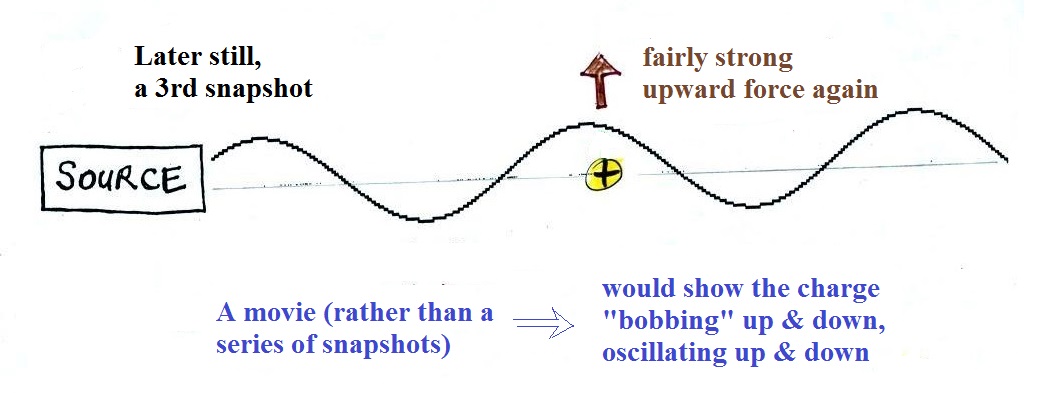
A 3rd snapshot taken a short time later. The + charge is now being pushed
upward again.
A movie of the + charge,
rather than just a series of snapshots, would show the charge
bobbing up and down much like a swimmer in the ocean would do as
waves passed by.
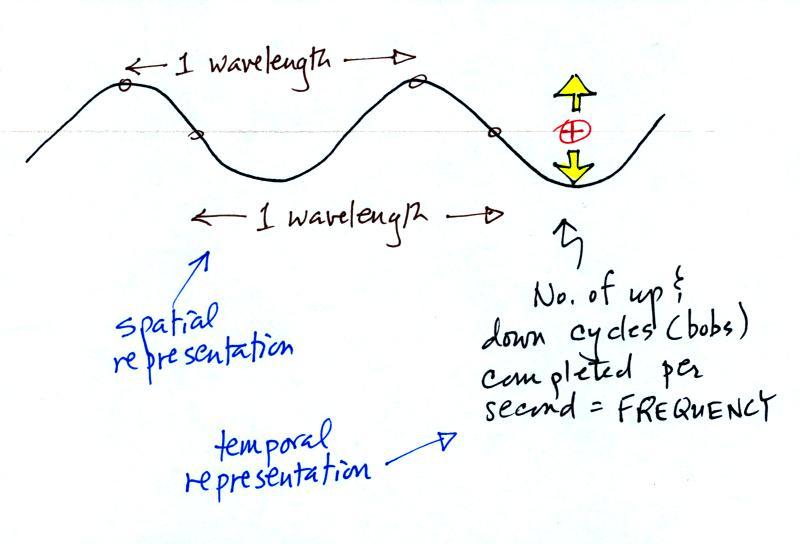
Wavelength and frequency
Just as light (which is EM radiation) comes in different colors,
there are types of EM radiation. A couple are sketched
below.
How would you describe the difference you see above in words?
The wavy pattern can be described spatially (what you would see at a particular
time if the EM radiation where spread out in a snapshot) in terms
of its wavelength, the distance between identical points
on the pattern.
Or you can describe the radiation temporally using the
frequency of oscillation (number of up and down cycles
completed by an oscillating charge per second). By
temporally we mean you look at one particular fixed point and look
at how things change with time.
The
following figure illustrates how energy can be transported
from one place to another (even through empty space) in the
form of electromagnetic (EM) radiation.
You add energy when you cause an
electrical charge to move up and down and create the EM
radiation (top left).
In the middle figure, the EM
radiation that is produced then travels out to the right (it
could be through empty space or through something like the
atmosphere).
Once the EM radiation encounters an electrical charge at
another location (bottom right), the energy reappears as the
radiation causes the charge to move. Energy has been
transported from left to right.
We'll quickly review a couple of figures and then
add a third with some new information
2 ways of describing or differentiating between
different types of EM radiation: wavelength & frequency
You can describe the radiation spatially using
the wavelength. In this case you're looking at the
EM radiation at different locations at one particular time.
Or you can describe the EM radiation temporally using the
frequency. Frequency is the number of up and down
cycles a charge would complete per second.
The following figure illustrates how energy can be
transported from one place to another (even through empty
space) in the form of electromagnetic (EM) radiation.
You add energy when you cause an
electrical charge to move up and down and create the EM
radiation (top left).
In the middle figure, the EM
radiation that is produced then travels out to the right (it
could be through empty space or through something like the
atmosphere).
Once the EM radiation encounters an electrical charge at
another location (bottom right), the energy reappears as the
radiation causes the charge to move. Energy has been
transported from left to right.
Wavelength, frequency, and energy
There's an important association between wavelength, frequency,
and the energy in EM radiation.
EM radiation can be created when you cause a charge to
move up and down. If you move a charge up and down
slowly (upper left in the figure above) you would produce long
wavelength radiation that would propagate out to the right at the
speed of light. If you move the charge up and down more
rapidly you produce short wavelength radiation that propagates at
the same speed.
Once the EM radiation encounters the charges at the right side
of the figure above the EM radiation causes those charges to
oscillate up and down. In the case of the long wavelength
radiation the charge at right oscillates slowly. This is low
frequency and low energy motion. The short wavelength causes
the charge at right to oscillate more rapidly - high frequency and
high energy.
These three characteristics: long
wavelength / low frequency / low energy go
together. So do short wavelength / high
frequency / high energy. Note that the two
different types of radiation both propagate at the same speed.
The electromagnetic spectrum
The EM spectrum is just a list of the different kinds of EM
radiation. A partial list is shown below.
In the top list, shortwave wavelength/high energy forms of EM
radiation are on the left (gamma rays and X-rays for
example). Microwaves and radiowaves are longer
wavelength/lower energy forms of EM radiation.
We will mostly be concerned with just ultraviolet light (UV),
visible light (VIS), and infrared light (IR). These are
shown on an expanded scale below. Note the micrometer
(millionths of a meter) units used for wavelength for these kinds
of light. The
visible portion of the spectrum falls between 0.4 and 0.7
micrometers. UV and IR light are both
invisible. All of the vivid colors shown above are just EM
radiation with slightly different wavelengths. When you see
all of these colors mixed together, you see white light.
I've tried to demonstrate colors mixing together to make white
light using laser pointers.
But it's too hard to get them adjusted so that the small spots
of colored light all fall on top of each other on the screen at
the front of the room. And even if you do the small spot of
light is so small that it's hard to see clearly in a large
classroom (you need to do the experiment on a piece of paper a few
feet away).
Here's the basic idea, you mix red green and blue light
together. You see white light were the three colors overlap
and mix in the center of the picture above. Doesn't it seem
odd that green and red mix to produce yellow?