Monday, October 28
Condensation nuclei and the formation of
dew, frost, haze, fog, and clouds
Here's a visual summary of a part of what we'll be
covering next.

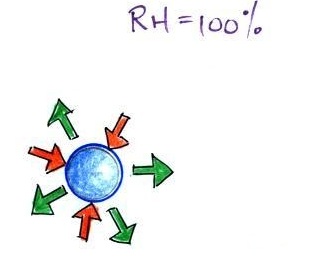
A variety of things can happen when you
cool air to the dew point and the relative humidity increases
to 100%. When moist air next to the ground
becomes saturated (RH reaches 100%) water vapor condenses onto
(or, in the case of frost, is deposited onto) the ground or
objects on the ground. This forms dew, frozen dew, and
frost.
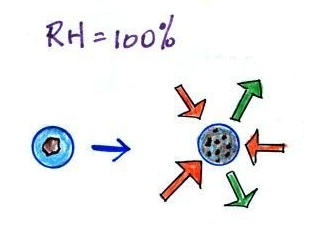
When air above the ground cools to the dew point, it is much
easier for water vapor to condense onto small particles in the air
called condensation nuclei. It would be much more difficult
for the water vapor to condense and form small drops of pure
water. Both the condensation nuclei and the small water
droplets that form on them are usually too small to be seen with
the naked eye. We can tell they are present because they
scatter sunlight and make the sky hazy. As humidity
increases dry haze turns to wet haze and eventually to fog.
We'll try to make a cloud in a bottle and you'll be able to better
appreciate the role that condensation nuclei play.
Condensation nuclei and the role
they play in cloud droplet formation
The air next to the ground cools during the night. Sometimes
it cools enough to reach the dew point. Water vapor
condenses onto objects on the ground and you find everything
covered with dew (or frost) the next morning. When this
happens in the air up above the ground you might think that water
vapor would simply condense and form little droplets. This
is not the case; we will find that small particles in the air
called condensation play an essential role in cloud (and fog)
formation.
it is much
easier for water vapor
to condense onto small particles
called condensation nuclei |
it
would be much harder
for water vapor
to just condense and form
small droplets of pure water
|
We won't go into
all of the details that follow in class, though they
aren't hard to figure out and understand. You're
free to just skip the details, but do remember that
particles make it much easier for cloud droplets and
clouds to form.
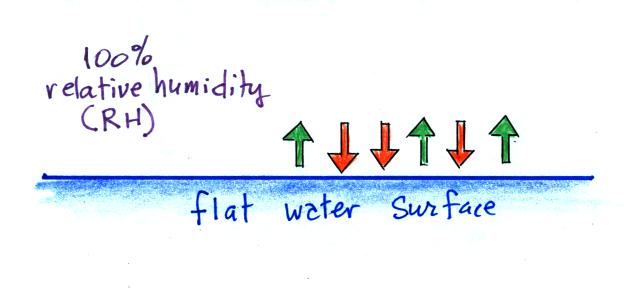
When the
air is saturated with water vapor (the relative humidity
is 100%) the rates of evaporation and condensation above a
flat surface of water will be
equal.
There's no real reason for picking three
arrows each of evaporation and condensation, the important
point is that they are equal when the RH is 100%.
It's hard for water vapor to
condense and form a small droplet of water because small
droplets evaporate at a very high rate. This is
known as the curvature effect and is illustrated below.
The surface of the smallest droplet
above at left has the most curvature and the highest rate
of evaporation (6 arrows). If a small droplet like
this were to form, it wouldn't stay around very
long. With it's high rate of evaporation it would
quickly evaporate away and disappear.
The middle droplet is larger and would stick around a
little longer because it does not evaporate as
quickly. But it too would eventually disappear.
The drop on the right is large enough that curvature no
longer has an effect. This drop has an evaporation
rate (3 arrows) that is the same as would be found over a
flat surface of water. A droplet like this could
survive, but the question is how could it get this big
without going through the smaller sizes with their high
rates of evaporation. A droplet must somehow reach
a critical size before it will be in equilibrium with its
surroundings.
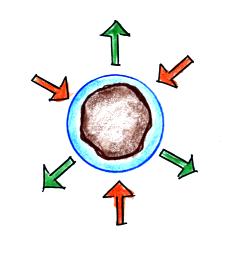
Particles in the air, cloud
condensation nuclei (CCN), make it much easier for cloud
droplets to form. The figure below explains why.
By condensing onto a particle, the water
droplet starts out large enough and with an evaporation
rate low enough that it is in equilibrium with the moist
surroundings (equal rates of condensation and
evaporation).
There are always lots of CCN (cloud
condensation nuclei in the air) so this isn't an
impediment to cloud formation. |
Now back to material that
we will cover in class.
The following information is from the bottom of page
91 in the ClassNotes.

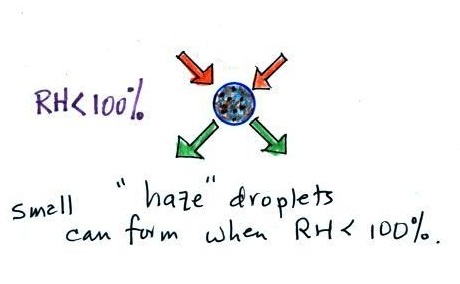
Note that condensation onto certain kinds of condensation nuclei
and growth of cloud droplets can begin even when the relative
humidity is below 100%. These are called hygroscopic
nuclei. Salt is an example; small particles of
salt mostly come from evaporating drops of ocean water.
I might try to show a video tape, not a
digital video but video recorded on a magnetic tape.
It will depend first of all on there being a VCR in the
classroom.
Here are some more
of the details that we won't cover in class.
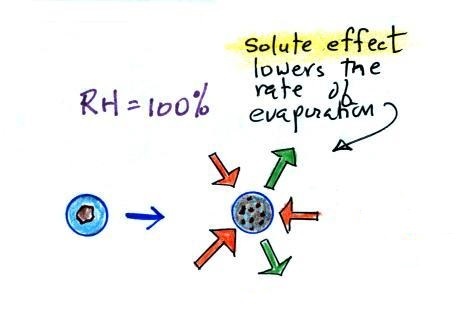
To understand how
condensation onto particles can begin even before the RH
has reached 100% we first need to learn about the solute
effect
|
|
solution
droplet
|
pure water
droplet
|
Water vapor condensing onto
the particle in the left figure dissolves the
particle. The resulting solution evaporates at a
lower rate (2 arrows of evaporation). A droplet of
pure water of about the same size would evaporate at a
higher rate (4 arrows in the figure at right).
Note the rates of condensation are equal in both figures
above. This is determined by the amount of
moisture in the air surrounding each droplet. We
assume the same moist (the RH is 100%) air surrounds
both droplets and the rates of condensation are
equal.
The next figure compares solution droplets that form
when the RH is 100% (left figure) and when the RH is
less than 100%.
|
|
the droplet is
able to grow
|
the droplet is
in equilibrium with its surroundings
even when the RH is less than 100%
|
The solution droplet will grow in the RH=100%
environment at left. You can tell the RH is less
than 100% in the figure at right because there are now
only 2 arrows of evaporation. But because the
solution droplet only has 2 arrows of evaporation it can
form and be in equilibrium in this environment.
|
We should remember
that much of what we see in the sky is caused by scattering of
light. There is a pretty good demonstration of light
scattering during a Startijenn concert shown below.
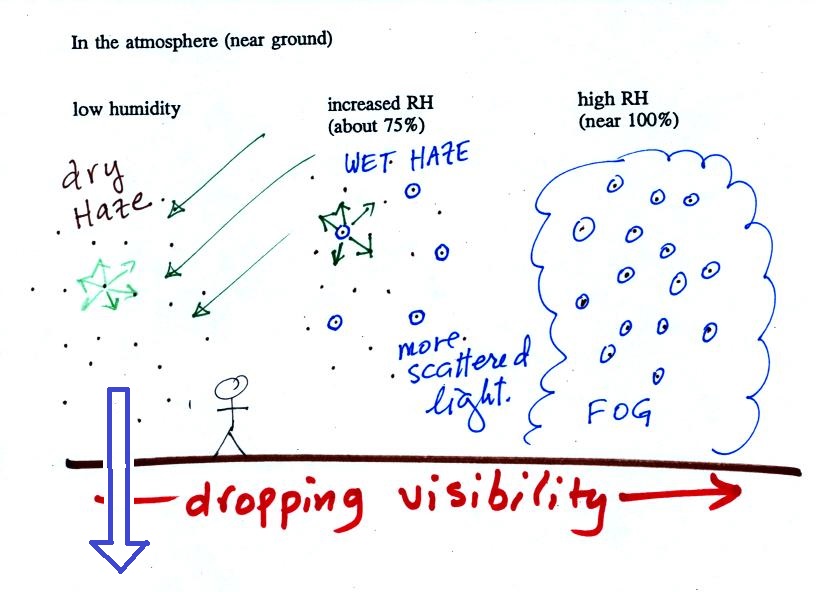
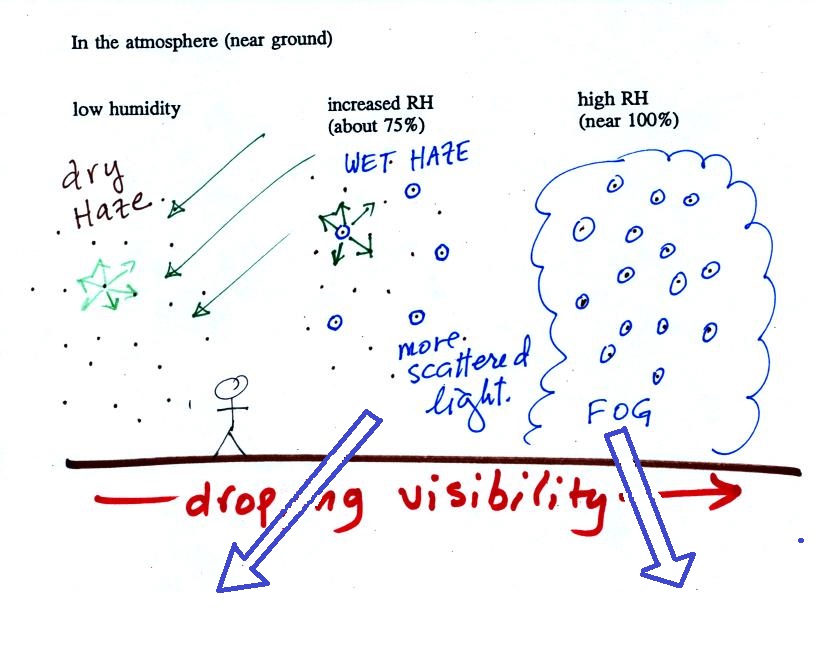
The
figure below is at the bottom of page 91 in the ClassNotes and
illustrates how cloud condensation nuclei and
increasing relative humidity can affect the appearance of the sky
and the visibility.
The air in the left most figure is relatively dry. Even
though the condensation nuclei particles are too small to be seen
with the human eye you can tell they are there because they
scatter sunlight. When you look at the sky you see the deep
blue color caused by scattering of sunlight by air molecules mixed
together with some white sunlight scattered by the condensation
nuclei. This changes the color of the sky from a deep blue
to a bluish white color. The more particles there are the
whiter the sky becomes. This is called "dry haze."
Visibility under these conditions might be anywhere from a few
miles up to a few tens of miles.
A photograph of fairly severe air pollution in
Paris that illustrates an extreme
case of dry haze (this is more common and
more severe in China and India). In Paris cars with
even numbered license plates weren't allowed into the city on
certain days of the week, odd numbers were banned on other
days. Public transportation was free for a short time to try
to reduce automobile use.
The middle picture below shows what happens when you drive from
the dry southwestern part of the US into the humid southeastern US
or the Gulf Coast. One of the first things you would notice
is the hazier appearance of the air and a decrease in
visibility. It isn't that there are more particles.
The relative humidity is higher, water vapor begins to condense
onto some of the condensation nuclei particles (the hygroscopic
nuclei) in the air and forms small water droplets. The water
droplets scatter more sunlight than just small particles
alone. The increase in the amount of scattered light is what
gives the air its hazier appearance. This is called "wet
haze." Visibility now might now only be a few miles.

|

|
Thin fog
(perhaps even wet haze)
with pretty good visibility
(source
of the image)
|
Thick fog
(visibility was less than 500 feet)
(source
of the image)
|

|
 |
Pictures of fog like
we sometimes get in Tucson (maybe once a year). The
picture at left is looking east from my house and was taken
early in the morning at the start of the spring semester in
2015. The picture at right is the view to the
west. Visibility was perhaps 1/4 mile.
|
Finally when the relative humidity increases to 100% fog forms and
water vapor condenses onto all the condensation nuclei. Fog
can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation
nuclei. That is part of the reason the Great London Smog of
1952 was so impressive. Visibility was at times just a few
feet!
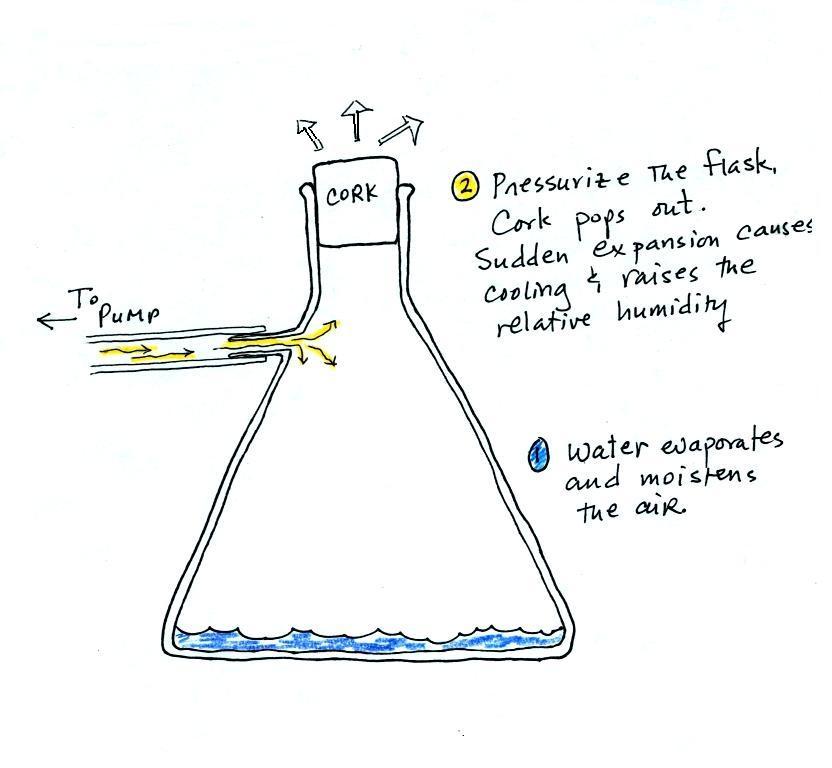
Making a cloud in a bottle
Cooling air (caused by sudden expansion) & increasing
relative humidity, condensation nuclei, and scattering of light
are all involved in this demonstration.
We used a
strong, thick-walled, 4 liter vacuum flask (designed
to not implode when all of the air is pumped out of
them, they really aren't designed to be
pressurized). There was a little water in the
bottom of the flask to moisten the air in the
flask. Next we pressurized the air in the
flask with a bicycle pump. At some point the
pressure blows the cork out of the top of the
flask. The air in the flask expands outward
and cools. This sudden cooling increases the
relative humidity of the moist air in the flask to
more than 100% momentarily and water vapor condenses
onto cloud condensation nuclei in the air.
I like it best when a faint, hard to see, cloud
becomes visible. That's because there is
something we can add to the demonstration that will
make the cloud much "thicker" and easier to see.
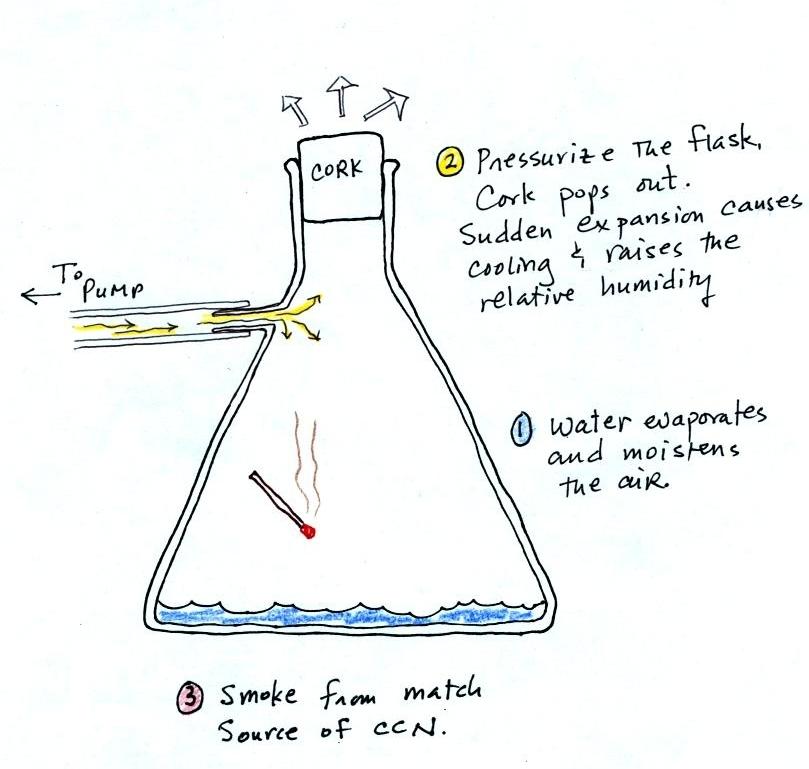
The demonstration was repeated an additional
time with one small change. A burning match was dropped
into the bottle. The smoke from the matches added lots of
very small particles, condensation nuclei, to the air in the
flask (you could see the swirls of smoke, the small particles
scattered light). The same amount of water vapor was
available for cloud formation but the cloud that formed this
time was quite a bit "thicker" and much easier to see. To
be honest the burning match probably also added a little water
vapor (water vapor together with carbon dioxide is one of the by
products of combustion).
I have found a couple of online versions of the
demonstration. The first
is performed by Bill Nye "The Science Guy" and is pretty similar
to the one done in class. The second
differs only in the way that is used to caused the sudden
expansion and cooling of the air (I didn't care much for the
music (probably your opinion of the music I play before class)
and I would recommend turning down the sound while watching the
video).
Mother Nature's version of the Cloud in a Bottle
demonstration
A
brush fire in this picture is heating up air and causing
it to rise. Combustion also adds some moisture and
lots of smoke particles to the air. You can see
that initially the rising air doesn't form a cloud (the
RH is still less than 100%). A little higher and
once the rising air has cooled enough (to the dew point)
a cloud does form. And notice the cloud's
appearance - puffy and not a layer cloud. Cumulo
or cumulus is the word used to describe a cloud with
this appearance. These kinds of fire caused clouds
are called pyrocumulus clouds. The example above
is from a
Wikipedia article fire-caused clouds.
The fire in this case was
the "Station Fire" burning near Los Angeles in August
2009. We sometimes see clouds like this in the summer
when lightning starts a fire burning in one of the nearby
forests. The pyrocumulus cloud caused by the fire is
sometimes the only cloud in the sky.
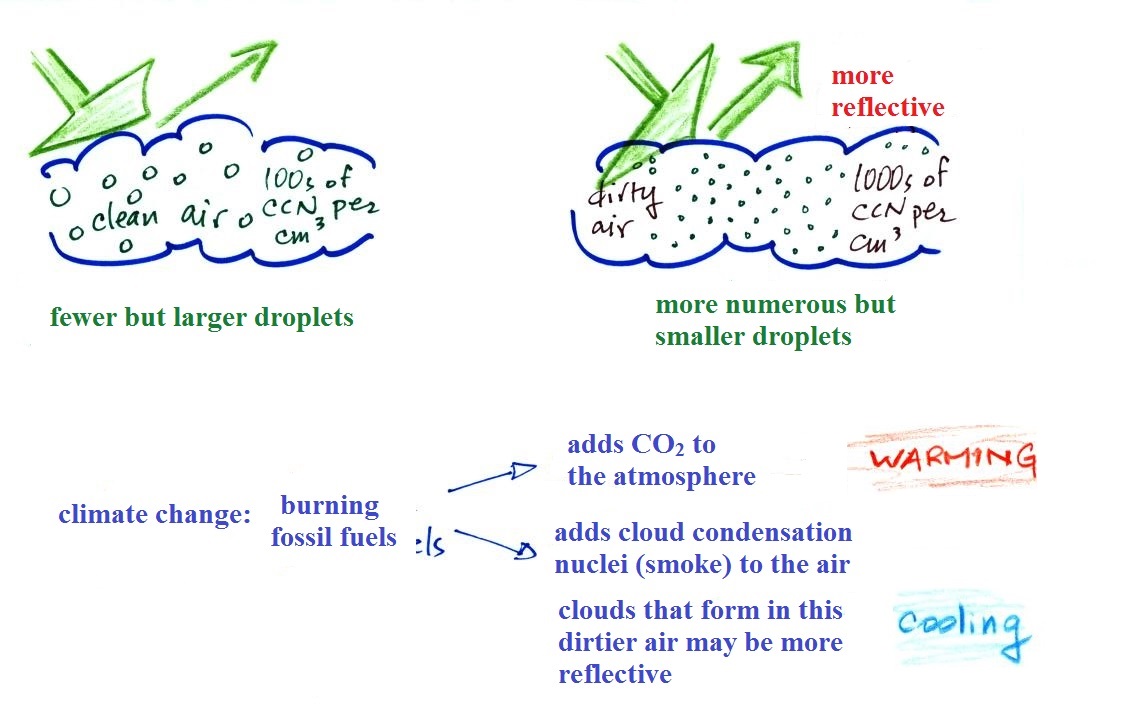
Clouds and climate change
This effect has some implications for climate change.
A cloud that forms in dirty air is composed of a large number
of small droplets (right figure above). This cloud is more
reflective than a cloud that forms in clean air, that is composed
of a smaller number of larger droplets (left figure).
Combustion of fossil fuels adds carbon dioxide to the
atmosphere. There is concern that increasing carbon
dioxide concentrations (and other greenhouse gases) will enhance
the greenhouse effect and cause global warming. Combustion
also adds condensation nuclei to the atmosphere (just like the
burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form,
might make the clouds more reflective, and might cause
cooling. There is still quite a bit of uncertainty about
how clouds might change and how this might affect climate.
Remember that clouds are good absorbers of far IR radiation and
also emit IR radiation. Clouds often raise nighttime low
temperatures.
Clouds are one of the best ways of cleaning the
atmosphere. This is something we mentioned earlier in the
semester and you're now in a position to understand it better.

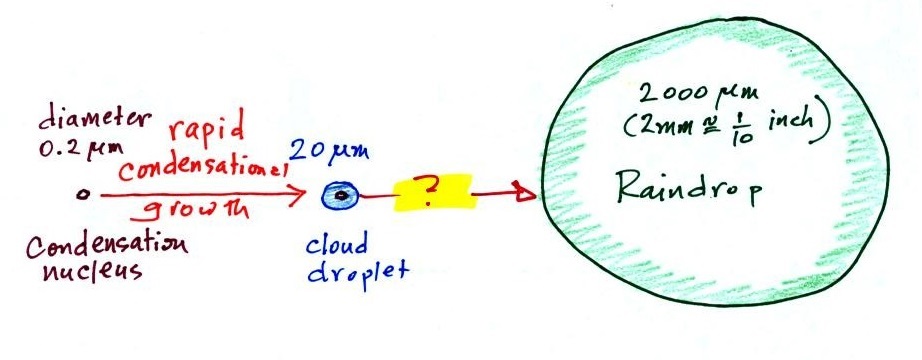
A cloud is composed of small
water droplets (diameters of 10 or 20 micrometers) that form on
particles ( diameters of perhaps 0.1 or 0.2 micrometers). The
droplets "clump" together to form a raindrop (diameters of 1000
or 2000 micrometers which is 1 or 2 millimeters), and the
raindrop carries the particles to the ground. A typical
raindrop can contain 1 million cloud droplets so a single
raindrop can remove a lot of particles from the air. You
may have noticed how clear the air seems the day after a
rainstorm; distant mountains are crystal clear and the sky has a
deep blue color. Gaseous pollutants can dissolve in the
water droplets and be carried to the ground by rainfall
also. We'll be looking at the formation of precipitation
in more detail next.
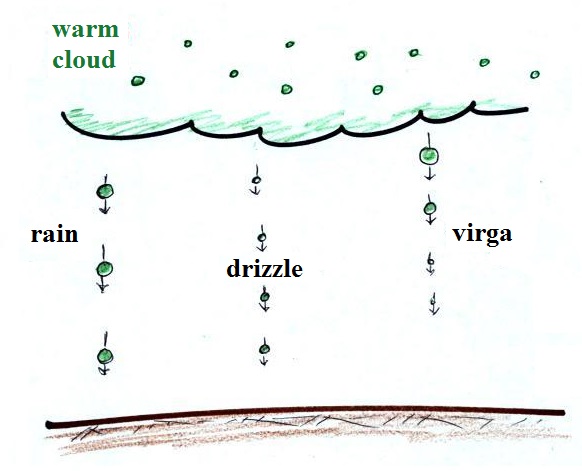
Formation of precipitation in clouds
Why is it so hard for clouds to make precipitation?
This figure shows typical sizes of cloud condensation nuclei
(CCN), cloud droplets, and raindrops (a human hair is about 50 μm
thick for comparison). As we saw in the cloud in a bottle
demonstration it is relatively easy to make cloud
droplets. You cool moist air to the dew point and raise
the RH to 100%. Water vapor condenses pretty much
instantaneously onto cloud condensation nuclei to form cloud
droplets. It would take much longer (a day or more) for
condensation to turn a cloud droplet into a raindrop. You
know from personal experience that once a cloud forms you don't
have to wait that long for precipitation to begin to fall.
Part of the problem is that it takes quite a bit more
water to make a 2000 μm diameter raindrop than it
does to make 20 μm diameter cloud
droplets . A raindrop is about 100 times
bigger across than a cloud droplet. How many droplets are
needed to make a raindrop? Before answering that question
we will look at a cube (rather than a sphere).
How many sugar cubes
would you need to make a box that is 4 sugar cubes on a
side?
It
would take 16 sugar cubes to make each layer
and there are 4 layers. So you'd need 64
sugar cubes. The key point is that we
are dealing with volumes, in the case of
a cube, volume is length x width x height.
The raindrop is 100 times wider, 100 times
bigger from front to back, and 100 times taller
than the cloud droplet. The raindrop has a
volume that is 100 x 100 x 100 = 1,000,000 (one
million) times larger than the volume of the cloud
droplets. It takes about a
million cloud droplets to make one average size
raindrop.
Precipitation-producing
processes
Fortunately there are two processes capable of quickly
turning small cloud droplets into much larger precipitation
particles in a cloud.
The collision coalescence process works in clouds that are
composed of water droplets only. This is often called
the "warm rain" process. Clouds like this are found in
the tropics (and very occasionally in the summer in
Tucson). We'll see that this is a pretty easy process to
understand.
The ice crystal process produces precipitation everywhere
else. This is the process that normally makes rain in
Tucson, even on the hottest day in the summer (summer
thunderstorm clouds are tall and grow into cold, below freezing,
parts of the atmosphere). Hail
and graupel often fall from
these summer storms; proof that the precipitation started out as
an ice particle). Thunderstorms also produce lightning and
later in the semester we will find that ice is needed to make the electrical charge
that leads to lightning.
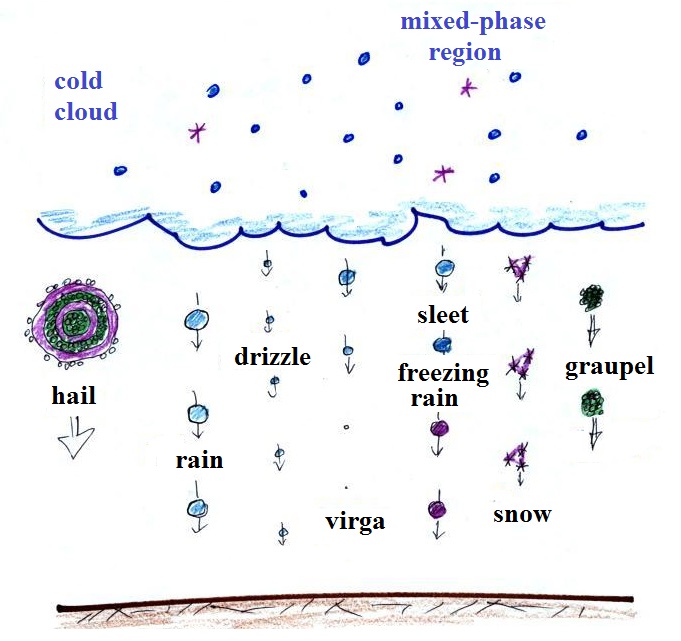
There is one part of ice-crystal process
that is a little harder to understand, but look at the
variety of different kinds of precipitation
particles (rain, snow, hail, sleet, graupel, etc) that can
result.
The Collision-Coalescence process
The collision coalescence process works in clouds that are
composed of water droplets only. Here's how it
works. The picture (found on page
101b in the ClassNotes) below shows what you
might see if you looked inside a warm cloud with just water
droplets:
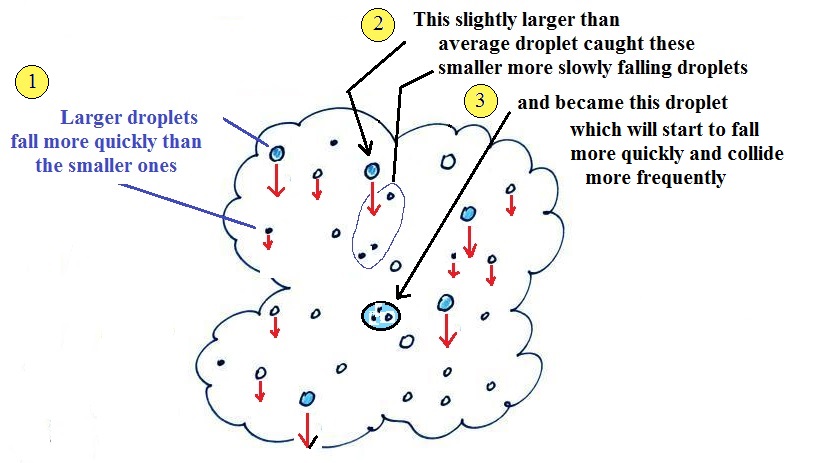
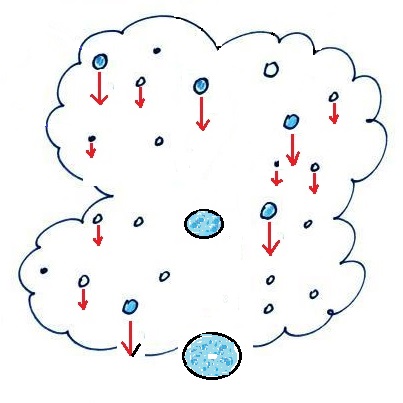
The collision coalescence
process works in a cloud filled with cloud droplets of
different sizes, that's critical. The larger
droplets fall faster than the small droplets. A
larger-than-average cloud droplet will overtake and collide
with smaller slower moving ones.
The bigger
droplets fall faster than the slower ones. They
collide and stick together (coalesce). The big drops
gets even bigger, fall faster, and collide more often with
the smaller droplets. This is an accelerating growth
process - think of a growing ball of snow as it rolls down
a snow-covered hill and picks up snow, grows, and starts
to roll faster and faster; or think of an avalanche
that gets bigger and moves faster as it travels downslope.
Very quickly a larger than average cloud droplet can grow to
raindrop size.
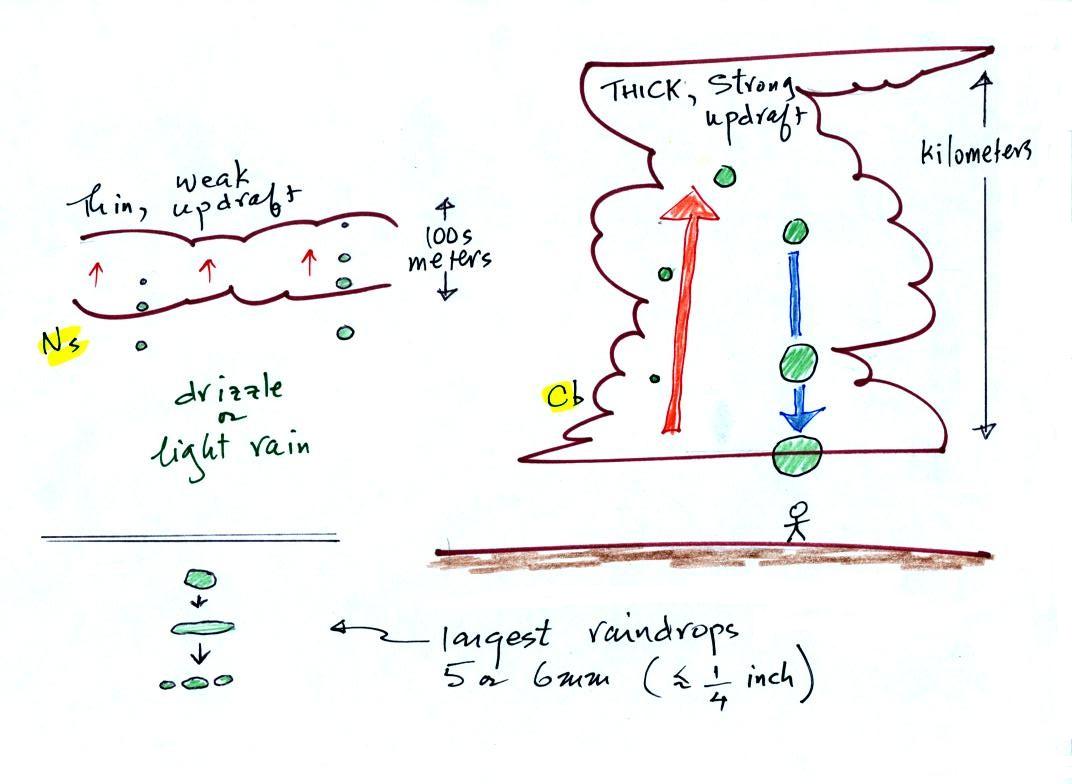
The figure shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns
clouds are thinner and have weaker updrafts than Cb
clouds. The largest raindrops fall from Cb clouds because
the droplets spend more time in the cloud growing. In a Cb cloud
raindrops can grow while being carried upward by the updraft (in
this case the smaller droplets are catching and colliding with
the larger droplets, but the end result is the same) and also
when falling in the downdraft.
Raindrops grow up to about 1/4 inch in diameter. When
drops get larger than that, wind resistance flattens out the
drop as it falls toward the ground. The drop quicly breaks
apart into several smaller droplets. Solid precipitation
particles such as hail can get much larger (an inch or two or
three in diameter).
And actually my sketch at lower left above isn't quite
accurate as this video of the breakup of
a 5 mm diameter drop of water shows.
The ice crystal process works in most locations most of the
time. Before we can look at how the ice crystal process
actually works we need to learn a little bit about clouds that
contain ice crystals - cold clouds.
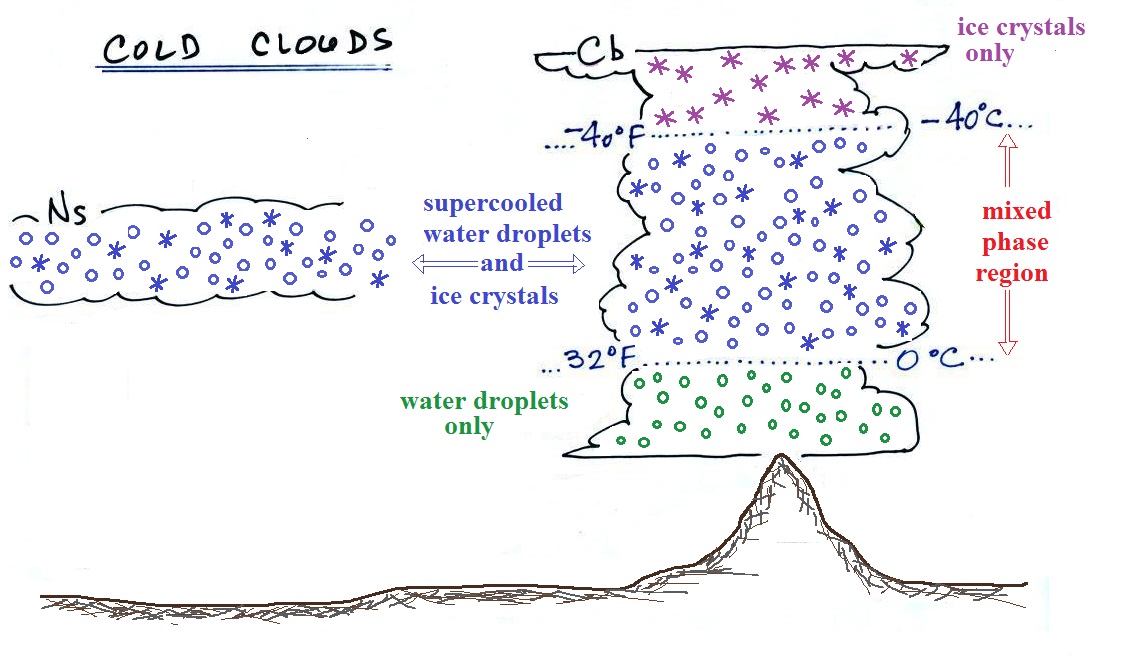
Cold clouds
The figure below shows the interior of a
cold cloud (see page
102a in the ClassNotes)
The bottom of the thunderstorm, Point 1, is warm enough
(warmer than freezing) to just contain water droplets. The
top of the thunderstorm, Point 2, is colder than -40 F (which,
coincidentally, is equal to -40 C) and just contains ice
crystals. The interesting part of the thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains
both supercooled water droplets (water that has been cooled to
below freezing but hasn't frozen) and ice crystals. This
is called the mixed phase
region. This is where the ice crystal process
will be able to produce precipitation. This is also where
the electrical charge that results in lightning is created.
Ice crystal nuclei
The supercooled water droplets in cold
clouds aren't able to freeze even though they have been
cooled below freezing. This is because it is much
easier for small droplets of water to freeze onto an ice
crystal nucleus (just like it is easier for water vapor to
condense onto condensation nuclei rather than condensing and
forming a small droplet of pure water). Not just any
material will work as an ice nucleus however. The
material must have a crystalline structure that is like that
of ice. There just aren't very many materials with
this property and as a result ice crystal nuclei are rather
scarce. In much of the mixed phase region there are
more supercooled water droplets than ice crystals.