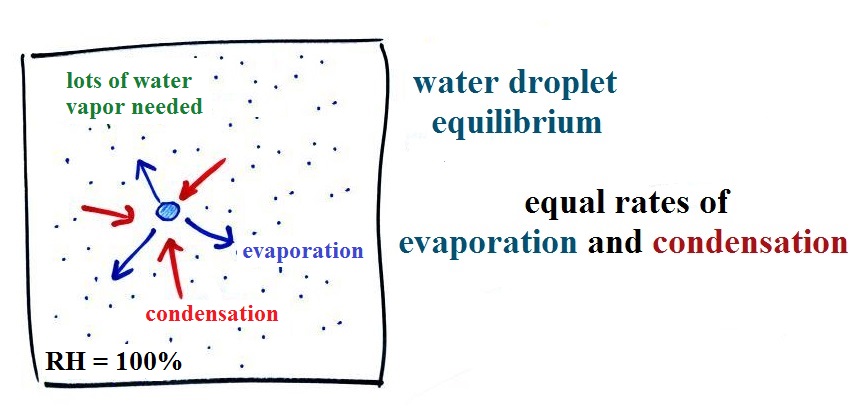
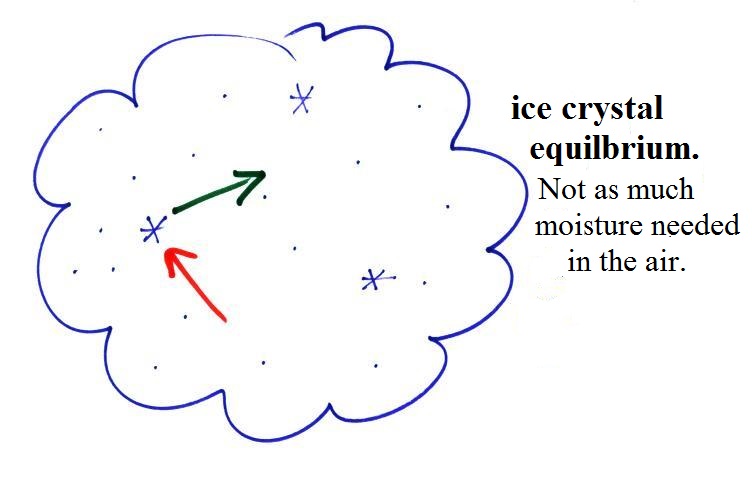
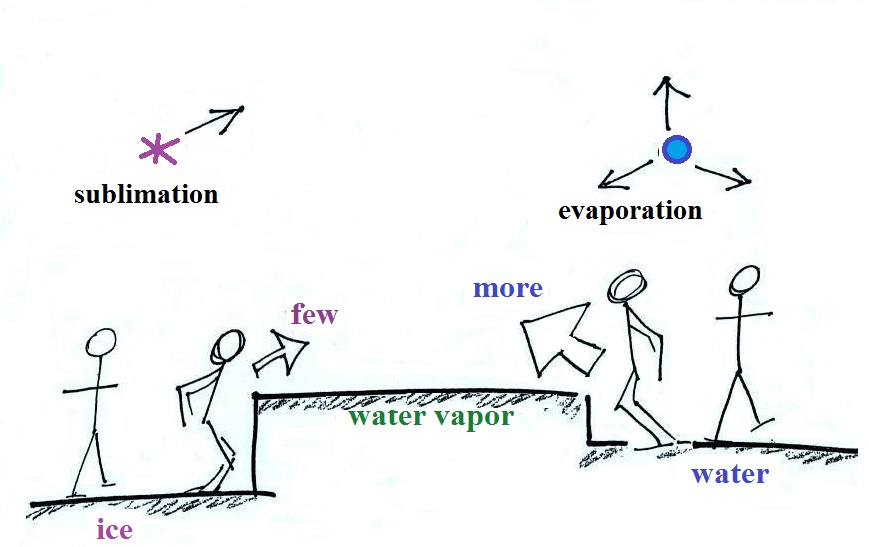
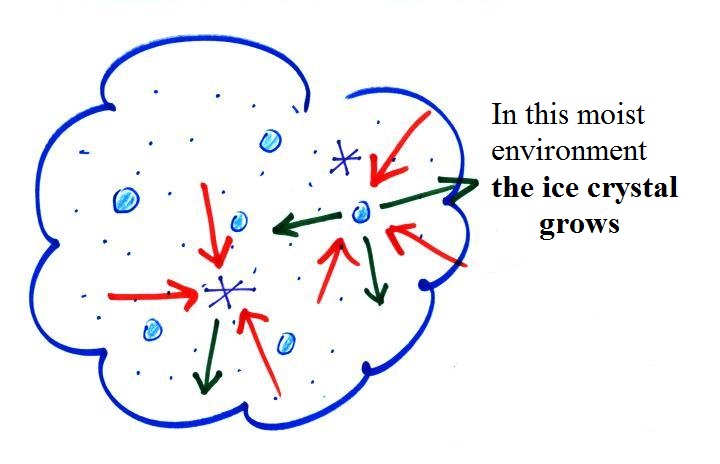
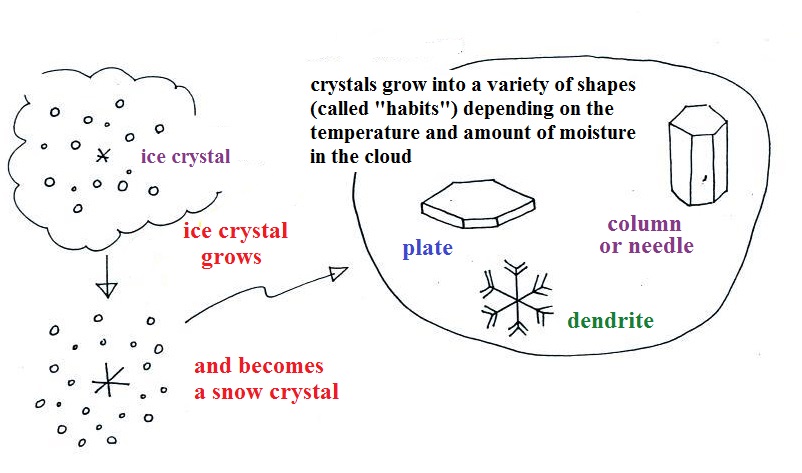
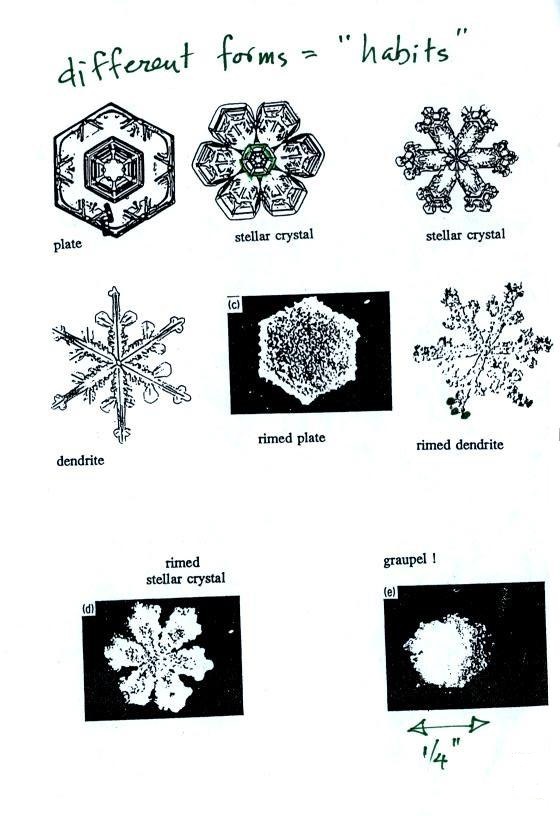
Here
are some actual photographs of snow crystals (taken with a
microscope). Snow crystals are usually 100
or a few 100s of micrometers in diameter (tenths of a
millimeter in diameter). That's visible but you'd need
a microscope to see the detail shown above.
You'll find some much better photographs and a pile of
additional information about snow crystals at www.snowcrystals.com.
Here's another
source of some pretty amazing photographs.
This Snow
Crystal Morphology Diagram (from SnowCrystals.com)
illustrates how the shapes of snow crystals depends on
temperature and the amount of moisture in the air.
Inside a cold cloud, once
the ice crystal process is underway
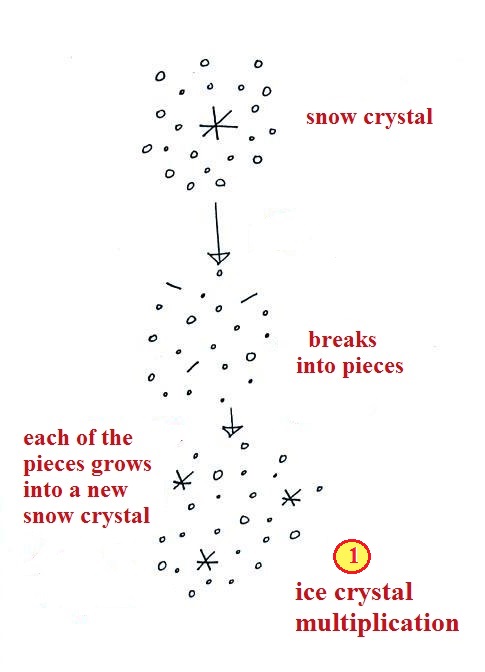
A variety of things can happen
once a snow crystal forms.
First it can break into
pieces, then each of the pieces can grow into a new snow
crystal. Because snow crystals are otherwise in rather
short supply, ice crystal multiplication is a way of
increasing the amount of precipitation that ultimately falls
from the cloud.
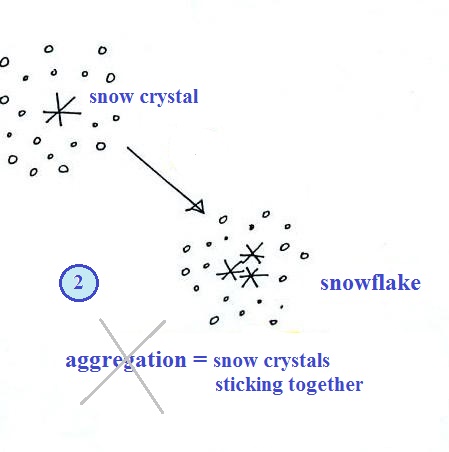
Snowflakes
Several snow crystals can collide and
stick together to form a snowflake. Snow crystals are
small, a few tenths of a millimeter across. Snowflakes
can be much larger and are made up of many snow crystals
stuck together. The sticking together or clumping
together of snow crystals is called aggregation. I
drew an X through the name in the figure because I
frequently forget the name of this process and don't expect
you to remember it either.
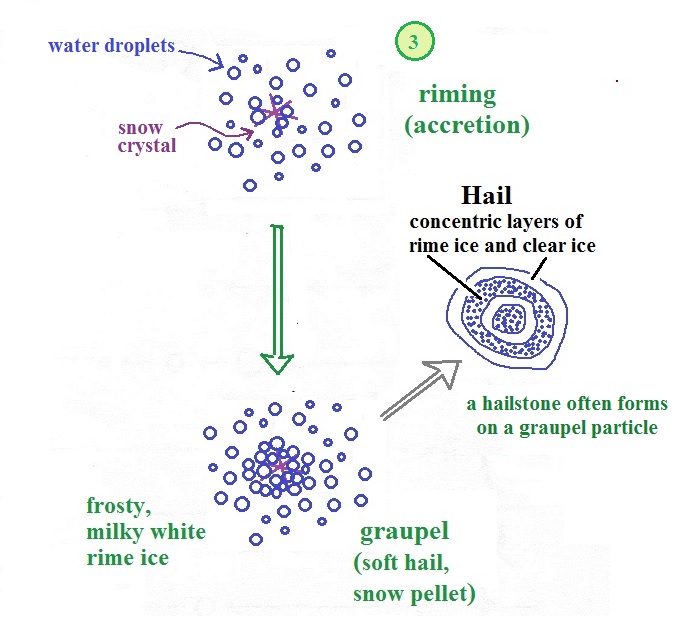
Riming (accretion) and graupel
(aka snow pellets & soft hail)
The next process and particle are something that I hope you
will remember.
Snow crystals can collide with
supercooled water droplets. The water droplets may
stick and freeze to the snow crystal. This process is
called riming or accretion (note this isn't called collision
coalescence even though it is the same idea). If a
snow crystal collides with enough water droplets it can be
completely covered with ice. The resulting particle is
called graupel. Graupel is sometimes mistaken for hail
and is called soft hail or snow pellets. Rime ice has
a frosty milky white appearance. A graupel particle
resembles a miniature snow ball. Or smaller finer
grained version of the shaved ice in a "snow cone."
Graupel particles often serve as the nucleus for a
hailstone. You'll find lots of pictures
on the internet .
Graupel is made of milky white
frosty rime ice. Sleet, we will find, is made of clear
ice. Here are some pictures to help you better
appreciate the differences in appearance.

Here's a snowball. It's
white and you can't see through it. It's made up
of lots of smaller crystals of ice. Graupel is
just a small snowball.
source
|

The ice in a snow cone is
basically the same. Lots of smaller chunks of
ice. The ice is frosty white (before you added
the flavored syrup).
source
|
Graupel vs sleet, rime
ice vs clear ice
Graupel is sometimes referred as snow pellets. Sleet is
sometimes called ice pellets.
|
clear transparent
sugar crystals
source
of this photograph
|
a frosty white sugar
cube made up
of many much smaller grains of sugar
|
Appreciating the differences in the appearance of clear ice
and rime ice.
Formation of hail
This figure (page
103b in the ClassNotes) gives you an idea of how hail
forms.
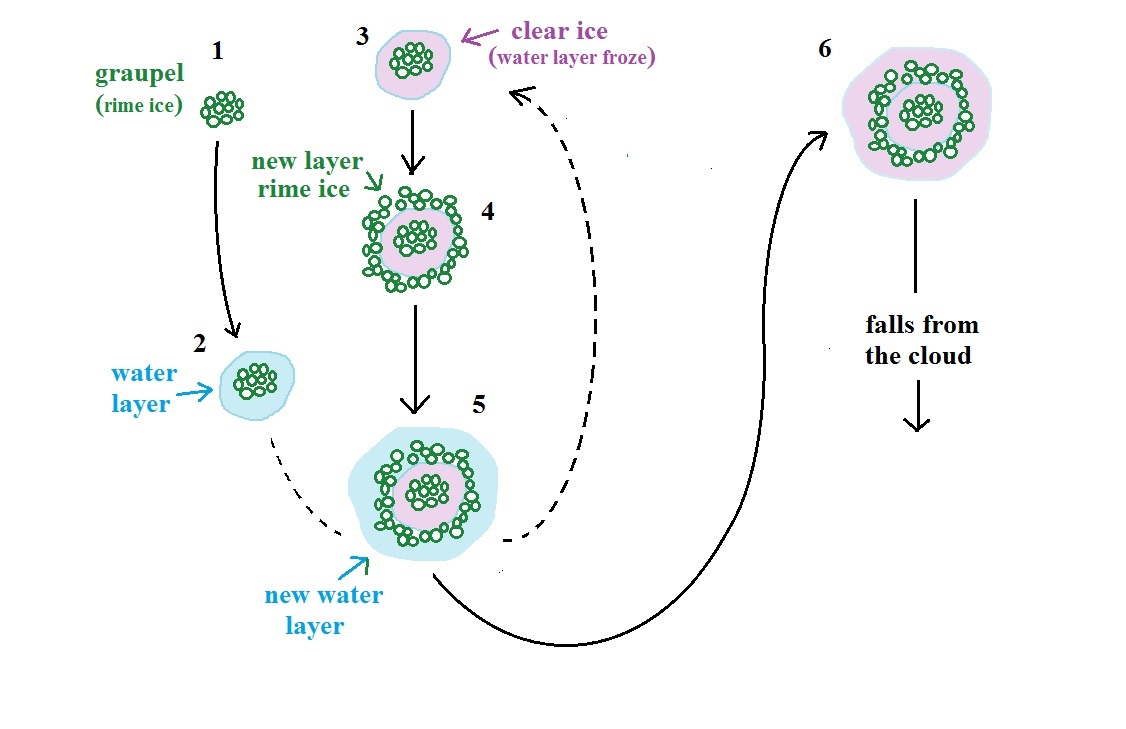
In the figure above a hailstone starts with a graupel
particle (Pt. 1, colored green to represent rime ice).
The graupel falls or gets carried into a part of the cloud
where it collides with a large number of supercooled water
droplets which stick to the graupel but don't immediately
freeze. The graupel gets coated with a layer of water
(blue) at Pt. 2. The particle then moves into a colder
part of the cloud and the water layer freeze producing a
layer of clear ice (the clear ice, colored violet, has a
distinctly different appearance from the milky white rime
ice), Pt. 3. In Tucson this is often the only example of
hail that you will see: a graupel particle core with a
single layer of clear ice (you can look through the clear
ice layer and see the graupel inside, here is a link
to some photographs from a Arizona Daily Star June,
2015 article). You'll also find
photographs if you search "hail
crossection photograph"
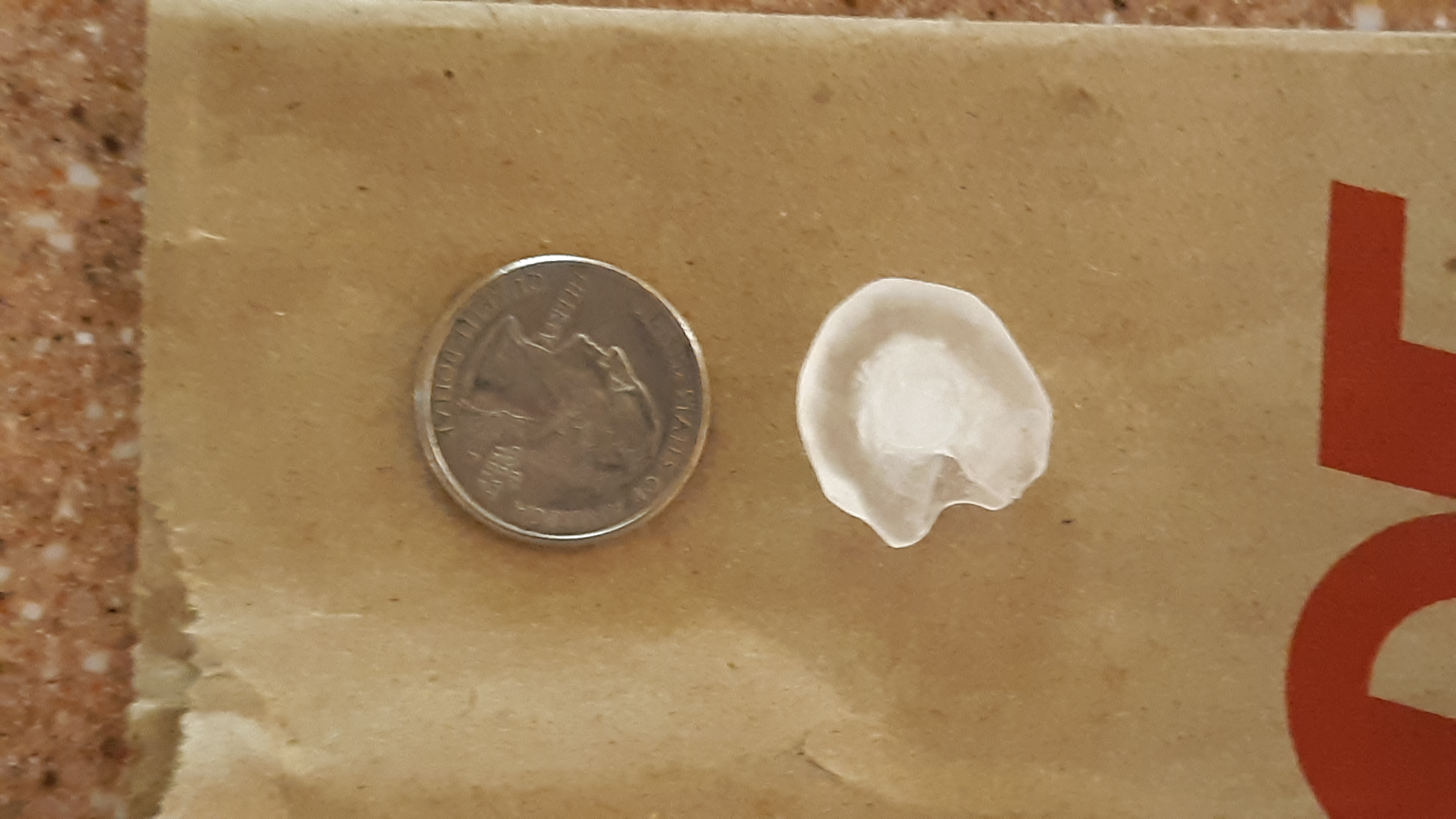
Here's a photograph of a hail stone that fell from a
thunderstorm cloud in midtown Tucson during the Fall 2018
semester (photo credit: Jim Dugan). It clearly shows the
frosty white graupel particle surrounded by a layer of clear
ice. It's pretty unusual to get hailstones this large in
Tucson.
In the
severe thunderstorms in the Central Plains, the hailstone
can pick up additional layers of rime ice and clear ice
and hailstones can be composed of many alternating layers
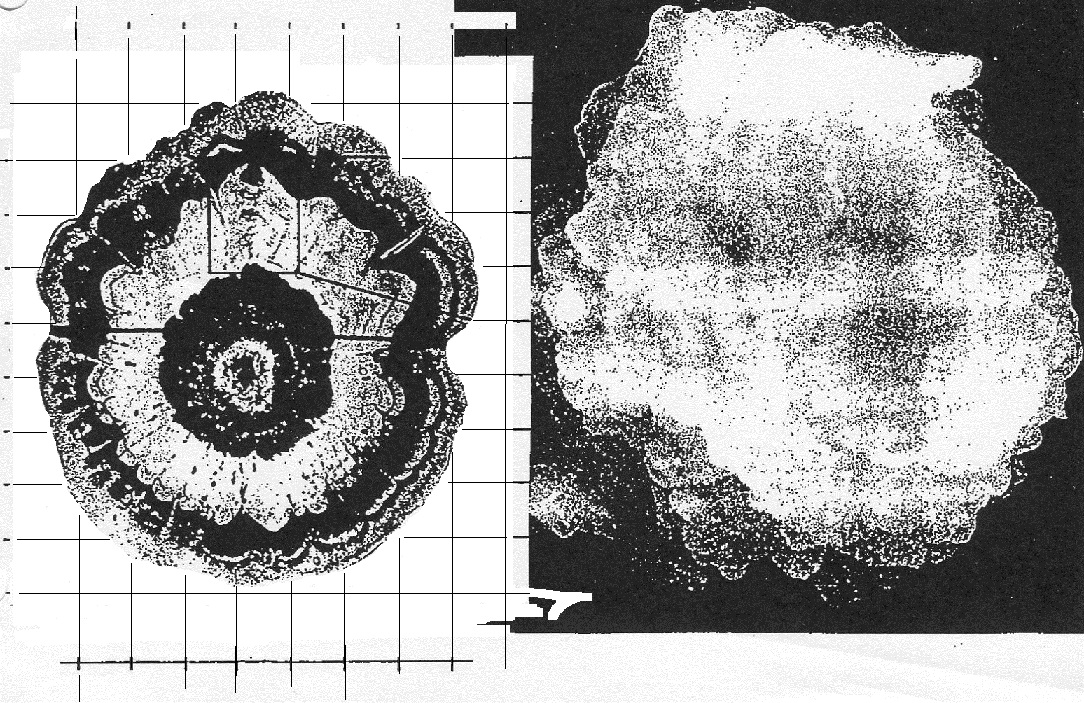
of rime and clear ice. An unusually large
hailstone (around 3 inches in diameter) has been cut in
half to show (below) the different layers of ice.
The picture below is close to actual size. If
something like this were to hit you in the head it would
split your skull open. Here's some pretty good video
of a hailstorm
in Phoenix.
Hail is produced in strong
thunderstorms with tilted updrafts. You would never
see hail (or graupel) falling from a nimbostratus cloud. Here
is a photo of a record setting 8" diameter hailstone
collected in South Dakota (it weighed nearly 2
pounds). I believe it is still the national record
holder. Click here
to see a gallery of images showing hail damage to
automobiles.
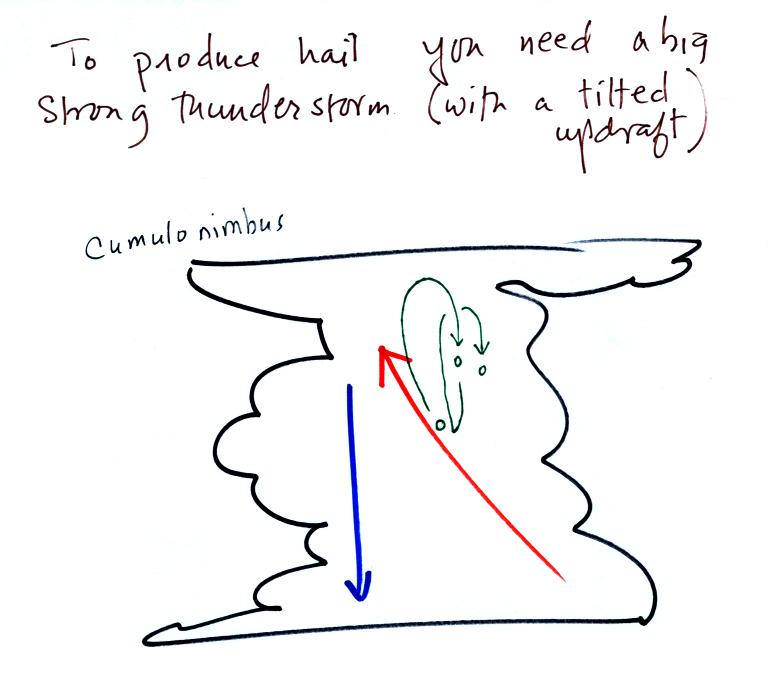
The growing hailstone can fall back into
the updraft (rather than falling out of the cloud) and be
carried back up toward the top of the cloud. In this way
the hailstone can complete several cycles through the interior
of the cloud. The article above mentions a supercell
thunderstorm. We will discuss these later in the
semester.
Types of precipitation
Finally on page
104a in the ClassNotes are illustrations of some of the
things that can happen once a precipitation particle falls
from a cloud. I've split this into two groups for
clarity.
Essentially all the rain that falls in Tucson is
produced by the ice crystal process. The left figure above
shows how this happens. A falling graupel particle or a
snow flake moves into warmer air and melts. The resulting
drops of water fall the rest of the way to the ground and would
be called RAIN.
In the middle picture graupel particles can survive the trip
to the ground without melting even in the summer. Many
people on the ground would call this hail but that wouldn't be
quite right. Graupel is less common in the winter because
it comes from thunderstorms and they don't form very often in
the winter. Snow can survive the trip to the ground in the
winter but not the summer. Snow does occasionally make it
to the valley floor in Tucson.
Sometimes the falling raindrops will evaporate before reaching
the ground. This is called VIRGA and is pretty
common early in the summer thunderstorm season in Arizona when
the air is still pretty dry. Lightning that comes from
thunderstorms that aren't producing much precipitation is called
"dry lightning" and often starts brush fires.
Rain will sometimes freeze before reaching the ground.
The resulting particle of clear ice is called SLEET.
FREEZING RAIN by contrast only freezes once it reaches the
ground. Everything on the ground can get coated with a thick
layer of ice. It
is nearly impossible to drive during one of these "ice
storms." Sometimes the coating of ice is heavy enough
that branches on trees are broken and power lines are brought
down (either by the weight of ice or falling tree limbs).
It sometimes takes several days for power to be
restored. Here's a gallery
of images taken after ice storms.