Thursday Feb. 02
The first optional assignment was collected today. They will be
returned next Tuesday. Answers are
now available online.
We should be able to begin returning graded 1S1P reports next week.
Now for a
little new material before the Practice Quiz. If this were a real
quiz you would have the full period for the quiz.
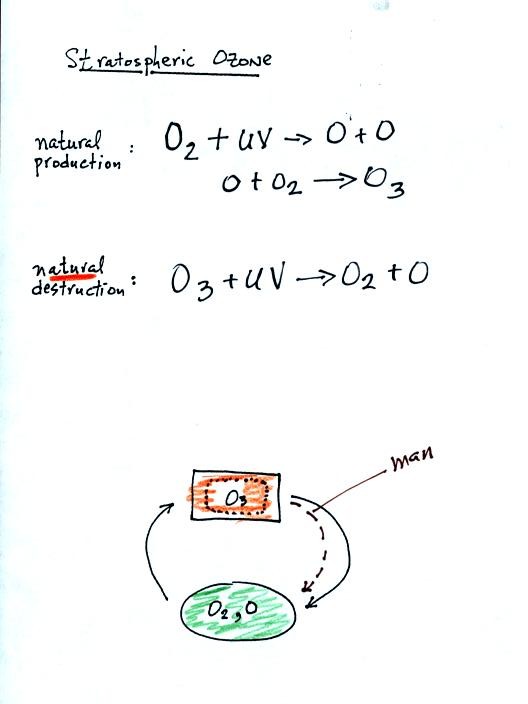
Molecular oxygen (O2) is split by UV light. One of the
two atoms
of oxygen can react with unsplit oxygen to make ozone. Ozone is
destroyed when it absorbs UV light. The O3 molecule is
split into
O and O2. Note O3 can also react with O to
make 2 molecules of
O2. This is another natural process of destruction for
ozone.
In the stratosphere an equilibrium would become established where the
rates of formation and destruction were equal. The ozone
concentration would not change. As we will see, man has added
additional processes of destruction that upset the natural
equilibrium. Adding new processes of destruction will cause the
ozone concentration to decrease somewhat.
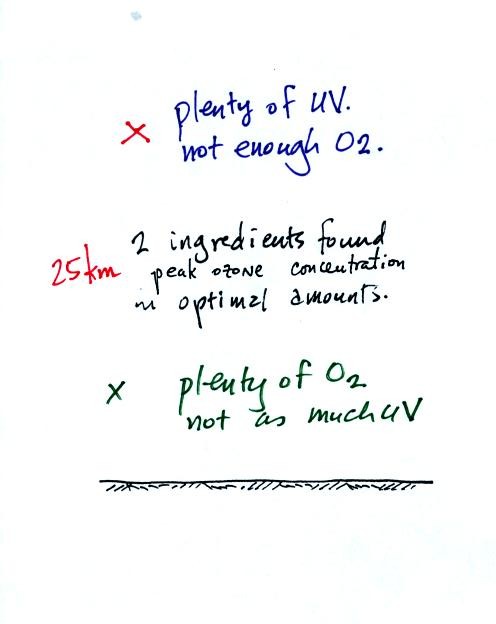
In middle latitudes, the optimal combination of UV light and molecular
oxygen are found near 25 km altitude. This is where peak ozone
concentrations are found.

If the concentration of ozone in the ozone layer were to decrease,
increased amounts of UV light would make it to the ground. Some
of the hazards associated with UV light exposure are given above.
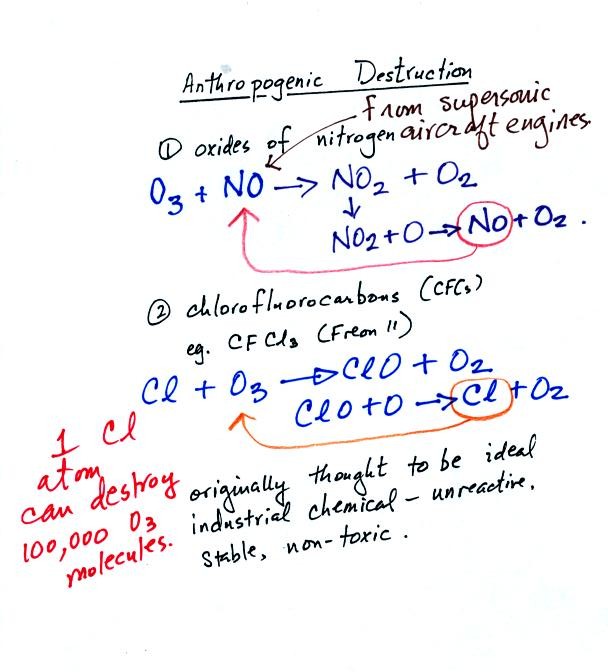
Nitric oxide (NO) such as would be emitted by the jet engines in
supersonic aircraft can destroy ozone. So can chlorine coming
from CFC molecules.
In the troposphere CFC molecules are stable and unreactive. They
can remain in the atmosphere for 50 to 100 years. This gives them
time to drift upward into the stable stratosphere. There the CFC
molecules are exposed to more intense UV light. This breaks atoms
of free chlorine off the CFC molecule. One Cl atom can react with
and destroy 100,000 ozone molecules before being removed somehow from
the stratosphere.
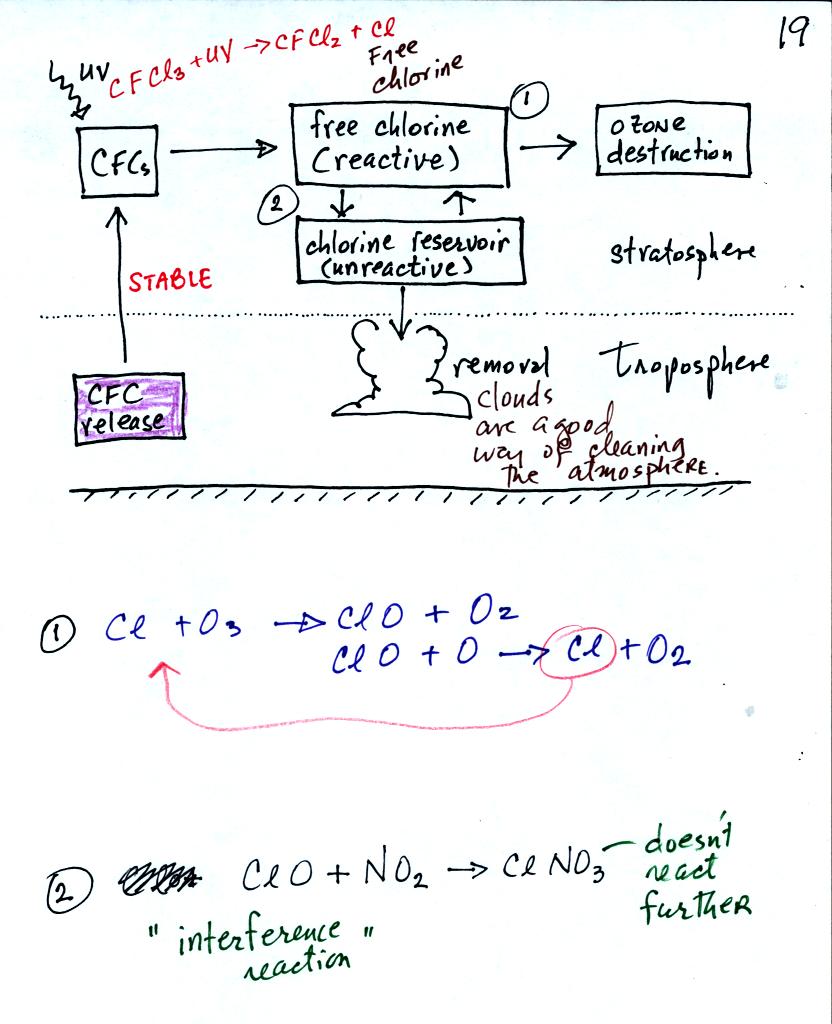
CFC molecules released in the troposphere can slowly drift upward into
the stratosphere. Then, as we have seen, chlorine (split off the
CFC molecules by intense UV light) can react with and destroy
ozone. Sometimes the Cl reacts with some other compound in the
stratosphere (such as NO2). This is an "interference
reaction",
it interferes with chlorine's ability to destroy ozone. Sometimes
the chlorine will be removed from the atmosphere altogether (clouds and
precipitation are the best way of doing this).
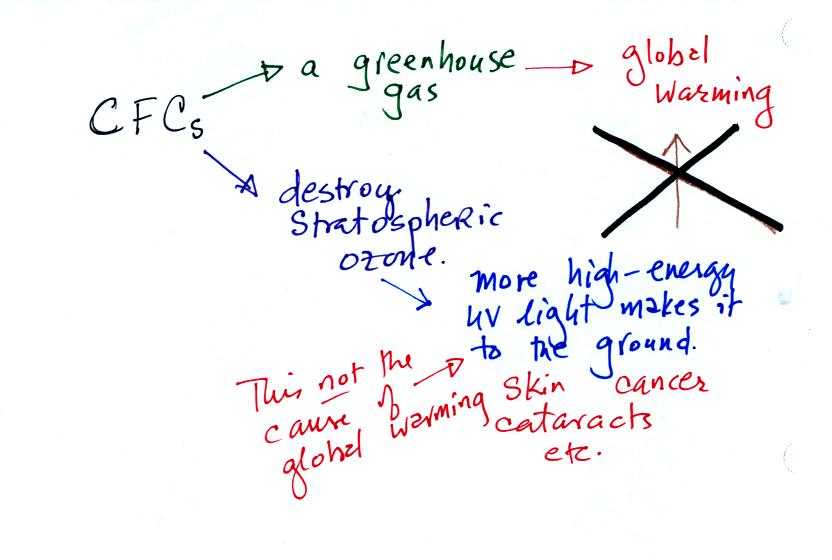
A common misconception is that thinning of the ozone layer is the cause
of global warming.
Part of the reason for this is that CFCs are both greenhouse gases and
capable of destroying ozone. The destruction of ozone allows more
UV radiation to reach the ground. It would seem logical that this
additional UV light would warm the surface. However there isn't
much UV in sunlight in the first place. If an extra little bit of
what already is a small amount of energy reaches the ground it isn't
going to cause much warming. The concern over this additional UV
light is that it can cause skin cancer, cataracts, etc.
WHen we get to Chapter 2 in this course (and we will eventually), we
will see that the greenhouse effect involves the propagation of IR
(infrared) light, not UV light, back and forth between the ground and
the atmosphere.
The
following material wasn't covered in class.
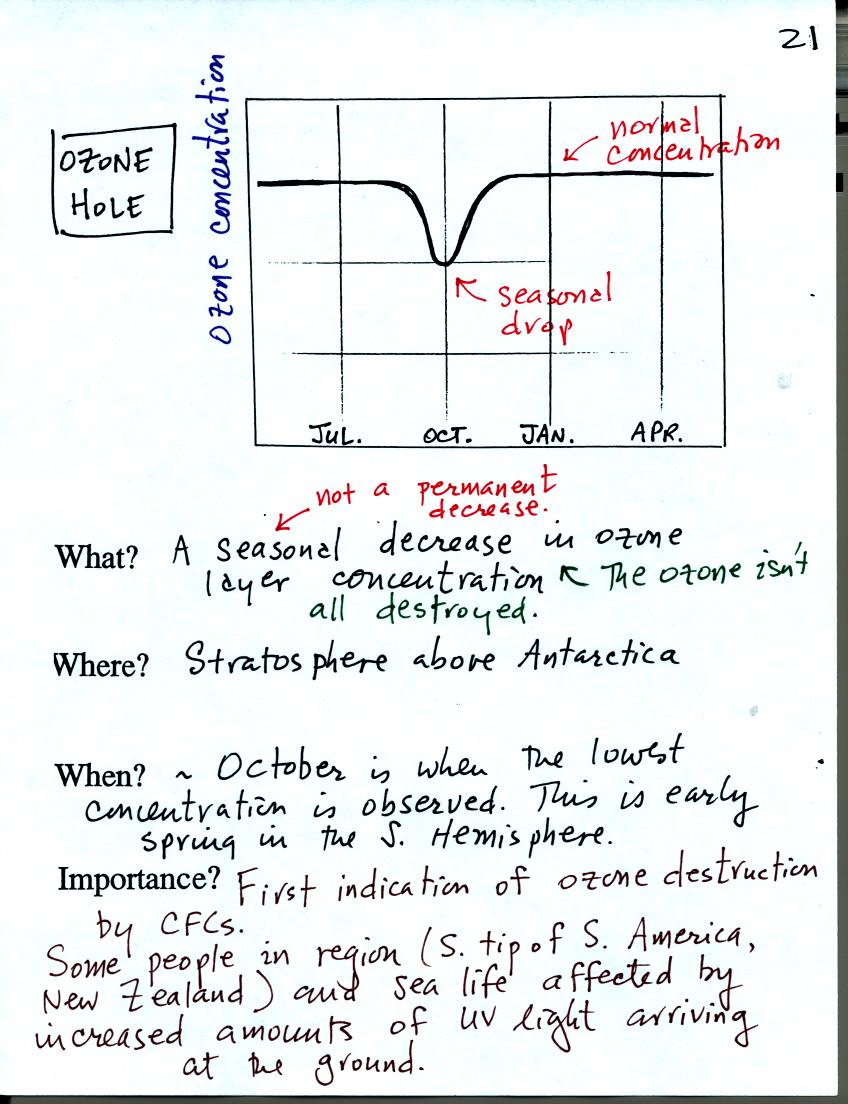
The ozone hole is a seasonal drop in ozone layer concentrations above
Antarctica. The "hole" forms around October every year.








