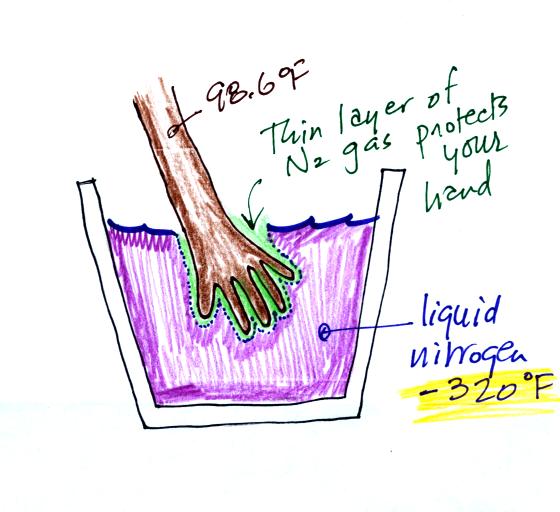
You can safely stick your hand in liquid
nitrogen for a fraction of a second. There is an
enormous temperature difference between your hand and the
liquid nitrogen which would ordinarily cause energy to leave
your hand at a dangerously high rate (which could cause your
hand to freeze solid). It doesn't feel particularly cold
though and doesn't feel wet. The reason why is that some
of the liquid nitrogen evaporates and quickly surrounds your
hand with a layer of nitrogen gas. This gas is a poor
conductor and insulates your hand from the cold for a very
short time (the gas is a poor conductor but a conductor
nonetheless) If you leave your hand in the liquid
nitrogen for even a few second it would begin to freeze.
That would cause irreparable damage.
A question came up in class about sticking you hand (or
maybe just the tip of one finger) into molten lead. I've
never seen it done and certainly haven't tried it
myself. But I suspected that you would first need to wet
your hand. Then once you stick it into the lead the
water would vaporize and surround your hand with a thin layer
of gas, water vapor. The water vapor is a poor conductor
just like the nitrogen and oxygen in air, and that protects
your hand, for a short time, from the intense heat.
Here's a
video.
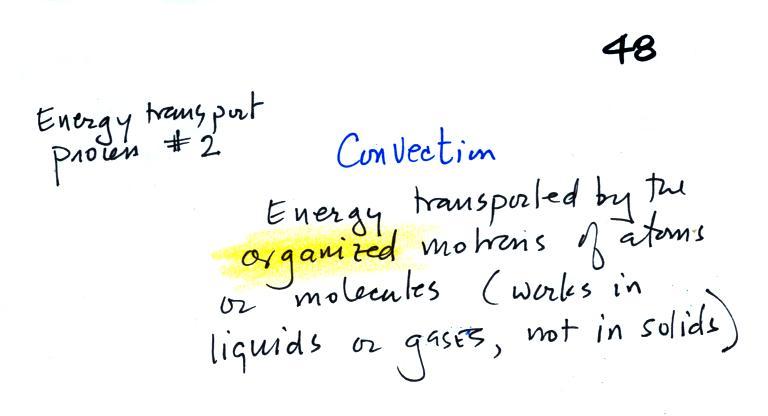
Wind chill is a good example of energy
transport by convection. As a matter of fact I'm hoping
that if I mention energy transport by convection that you'll
first think of wind chill. It is also a reminder that
our perception of cold is an indication of how quickly our
body is losing energy rather than an accurate measurement of
temperature. Today's wet, snowy, cold, windy weather is
a perfect time to experience wind chill for yourself.
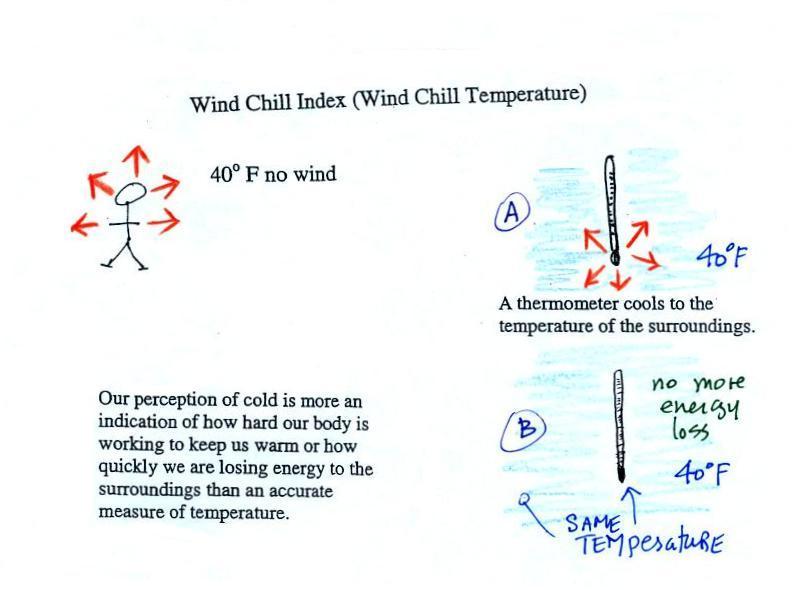
Your body works hard to keep its core
temperature around 98.6 F. If you go outside
on a 40 F day (calm winds) you will feel cool; your body is
losing energy to the colder surroundings (by conduction
mainly). Your body will be able to keep you warm for a
little while anyway (perhaps indefinitely, I don't
know). The 5 arrows represent the rate at which your
body is losing energy.
A thermometer behaves differently, it is
supposed to cool to the temperature of the surroundings.
Once it reaches 40 F and has the same temperature as the air
around it the energy loss will stop. If your body cools
to 40 F you will probably die.
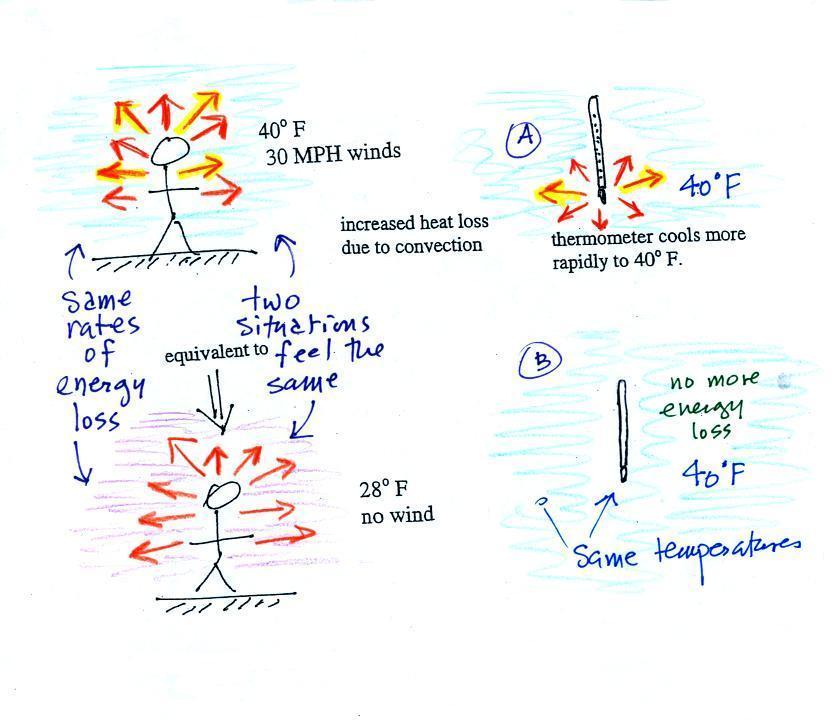
If you go outside on a 40 F day with 30
MPH winds your body will lose energy at a more rapid rate
(because convection together with conduction are transporting
energy away from your body). Note the additional arrows
drawn on the figures above indicating the greater heat
loss. This higher rate of energy loss will make it feel colder
than
a 40 F day with calm winds.
Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same
as a 28 F day without any wind. Your body is losing
energy at the same rate in both cases (9 arrows in both
cases). The combination 40 F and 30 MPH winds
results in a wind chill temperature of 28 F.
The thermometer will again cool to the temperature of its
surroundings, it will just cool more quickly on a windy
day. Once the thermometer reaches 40 F there won't be
any additional energy flow or further cooling. The thermometer would
measure 40 F on both the calm and the windy day.
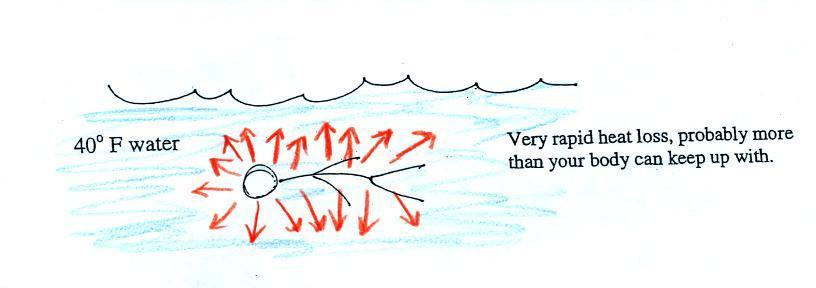
Standing outside on a 40 F
day is not an immediate life threatening situation.
Falling into 40 F water is, you'd last about 30 minutes
(you'd probably go unconscious before that and die by
drowning).
Energy will be conducted away from your
body more quickly than your body can replace it. Your
core body temperature will drop and bring on hypothermia.
One of the local TV stations reported that the Fire Department
treated two people for hypothermia today.
Be sure not to confuse hypothermia with hyperthermia
which can bring on heatstroke and is a serious outdoors risk
in S. Arizona in the summer.
We spent the remainder of the class period
looking at latent heat energy transport. This is the 3rd
energy transport process that we will cover.
If you had an object that you wanted to cool off quickly
you could blow on it. That might take a minute or two
(maybe more). Or you could stick it into some water,
that would cool it off pretty quickly because water will
conduct energy more rapidly than air. With a really hot
object immersed in water, you'd probably hear a brief sizzling
sound, the sound of boiling water. A lot of energy would
be taken quickly from the hot object and used to boil
(evaporate) the water. The cooling in this case takes
only a few seconds.
Latent heat energy transport is sometimes a little hard to
visualize or understand because the energy is "hidden" in
water vapor or water.

Latent heat energy transport is associated with changes of
phase (solid to liquid, water to water vapor, that sort of
thing) A solid to liquid phase change is melting, liquid to
gas is evaporation, and sublimation is a solid to gas phase
change. Dry ice is probably the best example of
sublimation. When placed in a warm room, dry ice turns
directly from solid carbon dioxide to gaseous carbon dioxide
without melting first. If you wash clothes and stick
them outside on a cold (below freezing) day they will
eventually dry. The clothes would first freeze but then
the ice would slowly sublimate away.
In each case above energy must be added to the material
changing phase. You can consciously add or supply the
energy (such as when you put water in a pan and put the pan on
a hot stove) or the phase change can occur without you playing
any role. In that case the needed energy will be taken
from the surroundings.
Here's the simplest example I can think of (this example wasn't mentioned in class)
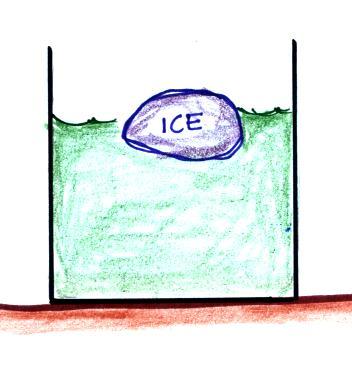
You put an ice cube in a
glass of warm water.
.
Once the ice had absorbed enough energy it would
melt. Energy taken from the water would cause the water
to cool. The energy that needed to be added to the ice
would be taken from the surroundings (the water) and would
cause the surroundings to cool.
Here's another example you should be very familiar with.
When you step out of the shower in the morning you're
covered with water. Some of the water evaporates.
It does so whether you want it to or not. Evaporation
requires energy and it gets that energy from your body.
Because your body is losing energy your body feels cold.

The object of this figure is
to give you some appreciation for the amount of energy
involved in phase changes. A 240 pound man (I have been
using Tedy
Bruschi as an example for several years) or woman
running at 20 MPH has just enough kinetic energy (if you could
capture it) to be able to melt an ordinary ice cube. It
would take 8 people running at 20 MPH to evaporate the
resulting ice water.
Phase changes can also go in the other
direction.

You can consciously remove energy from
water vapor to make it condense. You take energy out of
water to cause it to freeze (you could put water in a
freezer; energy would flow from the relatively warm
water to the colder surroundings). If one of these phase
changes occurs, without you playing a role, energy will be
released into the surroundings (causing the surroundings to
warm). Note the orange energy arrows have turned around
and are pointing from the material toward the
surroundings. It's kind of like a genie coming out of a
magic lamp. One Tedy Bruschi worth of kinetic energy is
released when water freezes to make a single ice cube.
Many genies, many Tedy Bruschis, are released when water vapor
condenses.
This release of energy into the surroundings and the
warming of the surroundings is a little harder for us to
appreciate because it never really happens to us in a way that
we can feel. Have you ever stepped out of an
air conditioned building into warm moist air outdoors and had
your glasses or sunglasses "steam up"? That never
happens to you (i.e. your body doesn't steam up) because your
body is too warm. However if it did you would feel
warm. It would be just the opposite of the cold feeling
when you step out of the shower or a pool and the water on
your body evaporates. You know how cold the evaporation
can make you feel, the same amount of condensation would
produce a lot of warming.

A can of cold drink will warm more quickly
in warm moist surroundings than in warm dry
surroundings. Equal amounts of heat will flow from the
warm air into the cold cans in both cases. Condensation
of water vapor is an additional source of energy and will warm
that can more rapidly. I suspect that the condensation
may actually be the dominant process.
The foam "cozy", "koozie",
or whatever you want to call it, that you can put around a can
of soda or beer is designed to insulate the can from the
warmer surroundings but also to keep water vapor in the air
from condensing onto the can.
We were out of time at this point. We really only
need to understand how energy can be transported from one
location to another in the form of latent heat. The
figure below, not shown in class,
tries to do that. I'll show this figure and another one
in class on Friday before we get started on electromagnetic
radiation, the 4th and most important energy transport
process.

You've just stepped out of the shower and
at Pt. 1 energy is being taken from your body and being used
to evaporate water. The water vapor, now containing the
energy from your body (Pt. 2), is free to move from the
bathroom to the kitchen where a cold can is sitting on a
table. Water vapor comes into contact with the cold can
and condenses at Pt. 3. The hidden latent heat energy in
the water vapor is released into the can and warms the drink
inside.
Energy has effectively been transported from your body in the
bathroom to a can in the kitchen.