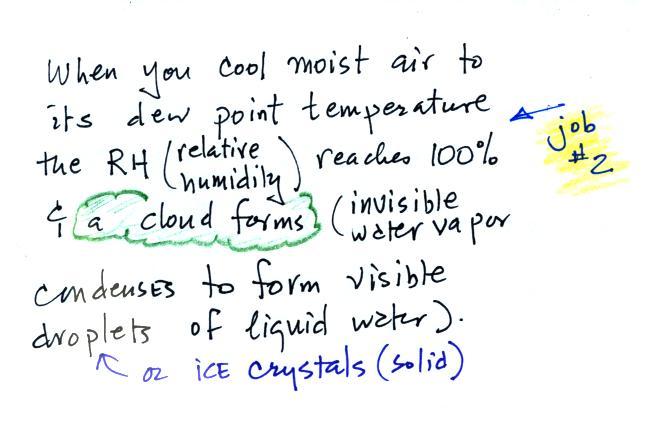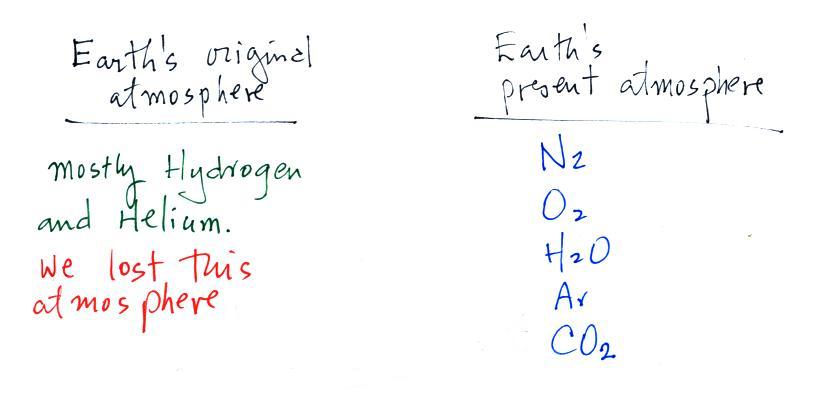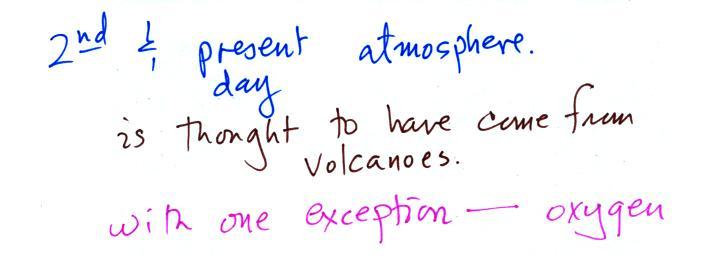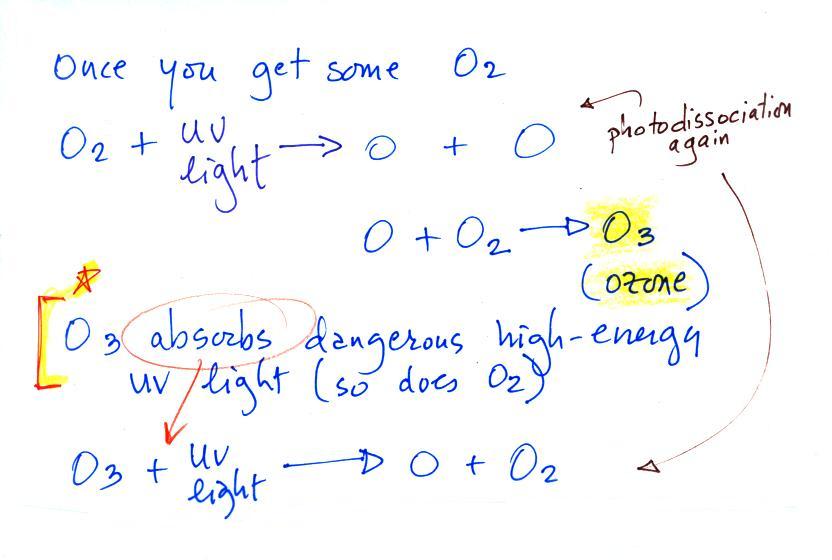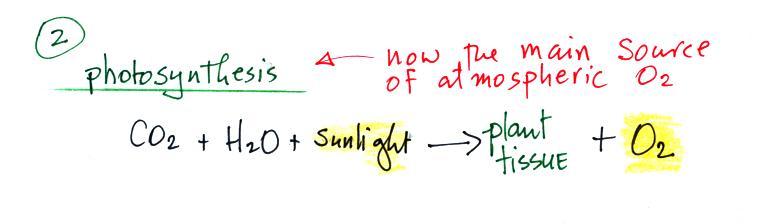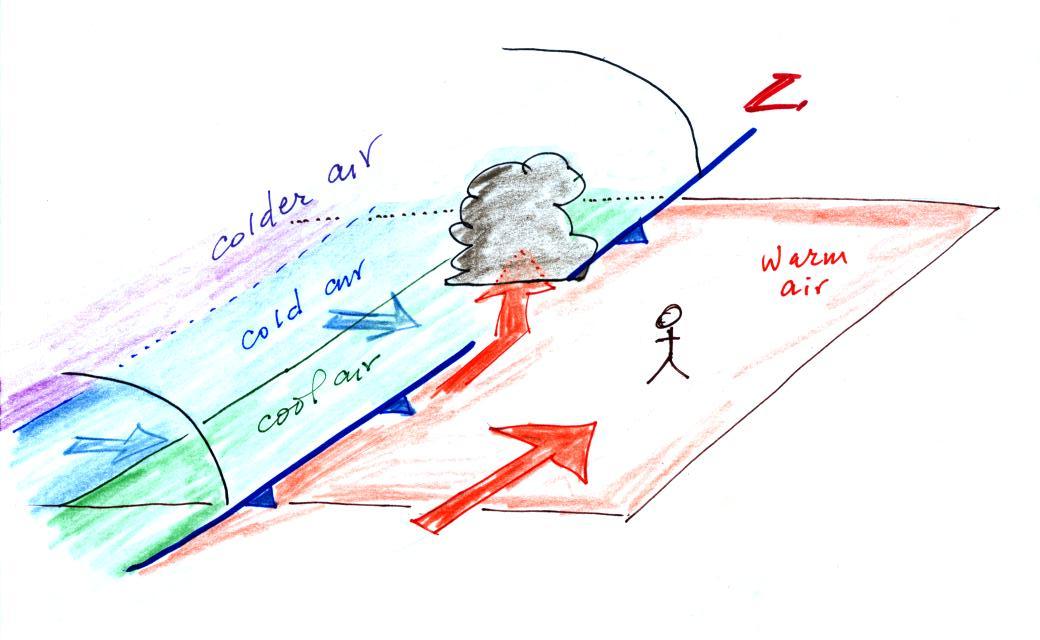
A cold front is a boundary
between warm and cold air. A cold front is found at
the leading edge of a bunch of cold air that is moving
into a region. A cold front is shown on on the
figure above and on surface weather maps using a blue line
with "points" on it. The points identify it as a
cold front and show what direction the front is moving.
About this time yesterday a cold front was located in western Arizona and was headed toward Tucson. At that time we were in the warm air found ahead of the front (figure above). The front passed through during the night and was accompanied by some gusty winds and light rain showers.
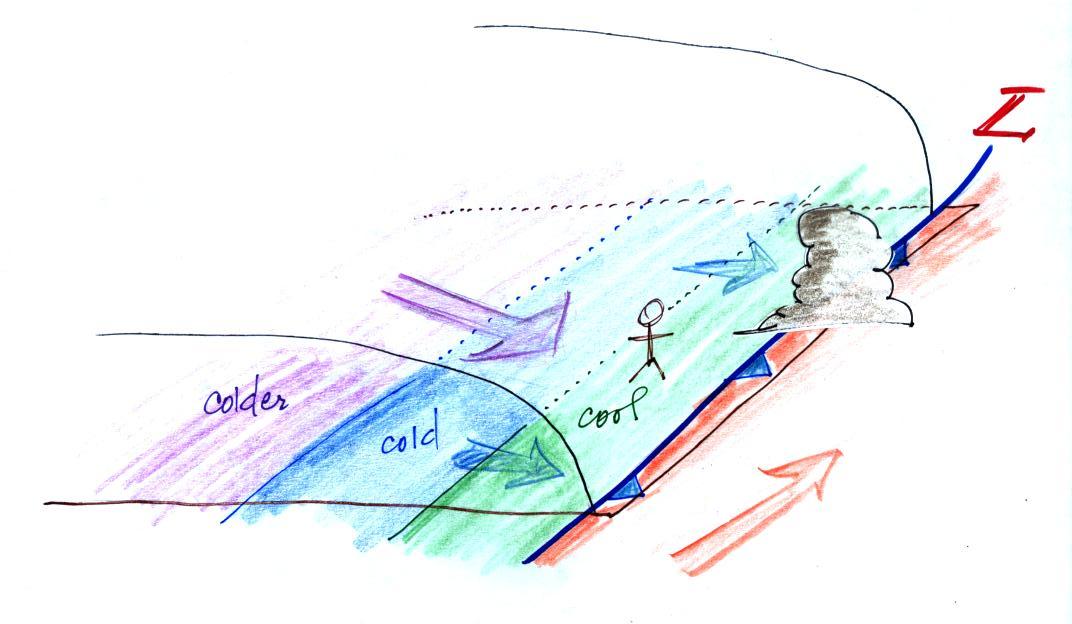
About this time yesterday a cold front was located in western Arizona and was headed toward Tucson. At that time we were in the warm air found ahead of the front (figure above). The front passed through during the night and was accompanied by some gusty winds and light rain showers.