Monday Jan. 14, 2013
click here to download today's notes
in a more printer friendly format
Mumford and Son and "I Will Wait"
before class this afternoon.
The cold weather persists. It meant the end of my last
year's tomatoes, though they usually don't last nearly this long
and I need the room for next year's garden. The tomato seeds
that I planted a week or so ago have germinated. I keep them
in my office because it's quite a bit warmer than my rental house.

|
|
Last summer's tomatoes
(killed by the cold weather)
|
Next summer's tomatoes
(growing in my office)
|
I handed out 10 more sets of Expt. #1 materials. I'll bring
another one or two on Wednesday but that will be the last call for
Expt. #1.
Have a look at the Report Signup lists and see if your name is
there. One of the signup sheets went missing last Friday and
I wasn't able to copy those names onto the online signup
lists. So if you're name isn't listed please let me know.
At the top of the list below are the 5 most abundant gases in
the atmosphere. Several more important trace gases, new
information, were added to the bottom of the
figure. Trace gases are gases found in low concentrations
(and often the concentrations vary with time and location).
Low concentrations doesn't mean they aren't important, however.

Carbon monoxide, nitric oxide, nitrogen dioxide,
ozone, and sulfur dioxide are some of the major air
pollutants. We'll cover 3 of these in more detail this
week.
Water vapor, carbon dioxide, methane, nitrous oxide (N2O = laughing gas),
chlorofluorocarbons, and ozone are all greenhouse gases.
Increasing atmospheric concentrations of these gases are
responsible for the current concern over climate change and
global warming. We'll discuss this topic and learn more
about how the greenhouse effect actually works later in the
course.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i)
Ozone in the stratosphere (a layer of the atmosphere between
about 10 and 50 km altitude) is beneficial because it absorbs
dangerous high energy ultraviolet (UV) light coming from the
sun. Without the protection of the ozone layer, life as
we know it would not exist on the surface of the earth.
It was only after ozone started to buildup in the atmosphere
that life could move from the oceans onto land.
Chlorofluorocarbons are of concern in the atmosphere because
they destroy stratospheric ozone.
(ii)
In the troposphere (the bottom 10 kilometers or so of the
atmosphere and where we live) ozone is a pollutant and is one
of the main ingredients in photochemical smog.
(iii) Ozone is also a
greenhouse gas.
Air Pollution is a
serious health hazard in the US and around the globe (click
here to download a
copy of the information below). The
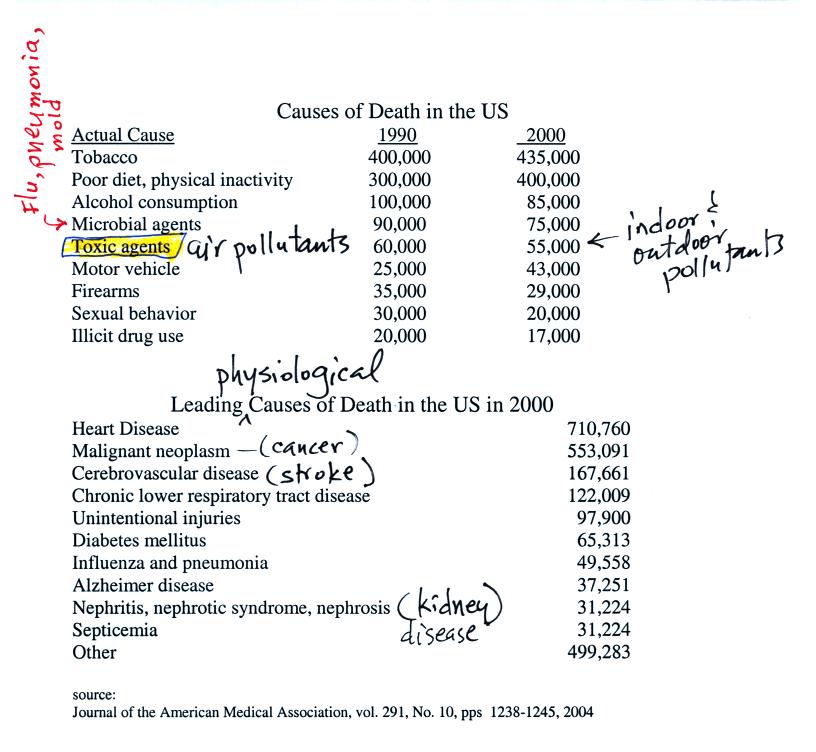
lists below give some idea of how serious a threat it is.
The top list shows the external or environmental agent that causes
death. The second list is the internal body function that
ultimately leads to your demise. Keep in mind that many of
these numbers are difficult to measure and some may contain a
great deal of uncertainty. The row that is highlighted,
toxic agents, contains estimates of deaths caused by indoor and
outdoor air pollution, water pollution, and exposure to materials
such as asbestos and lead both in the home and at the work
place. It is estimated that 60% of the deaths are due to
exposure to particulate matter, something that we will examine in
a little more detail late next week.

.
I'm not sure how the researchers determine that 150,000 people are
killed by climate change every year.
The Blacksmith
Institute listed the Top 10 polluted places in the
world in a 2007 report. The report has received a lot of
worldwide attention. If you go to this address (click on
2007 at the top left edge of the page) you can view the report
online or download and print a copy of the report. This is
just in case you are interested (click on some of the other years
also if you do go to the site). And note they are concerned
with all types of pollution, not just air pollution.
You may have heard of the record setting levels of air
pollution currently affecting Beijing, China. Here's a
link to a pretty good collection of photographs. Much
of this is particulate pollution which is something we'll cover
later this week or next week. In addition to being a health
hazard, particulates can have a dramatic effect on visibility.
We'll start our section on air pollutants with carbon
monoxide. You'll find additional information on
carbon monoxide and other air pollutants at the Pima County
Department of Environmental Quality website and also at the
US Environmental Protection Agency
website.
We will mostly be talking about carbon monoxide found outdoors,
where it would rarely reach fatal concentrations. CO is a
serious hazard indoors also where it can (and does) build up to
deadly concentrations (several
people
were
almost
killed
in
Tucson
in
December 2010 for example)
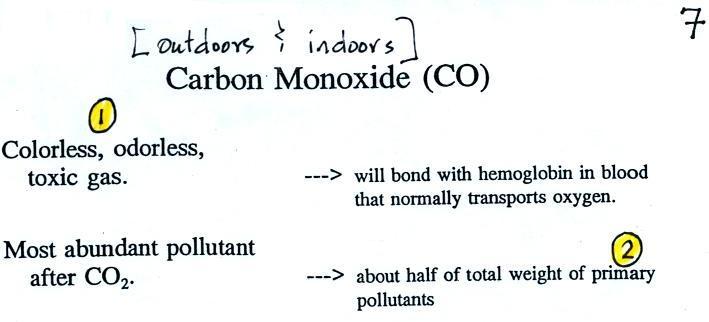
Carbon monoxide is insidious, you can't smell it or see it and
it can kill you (Point 1).
Once
inhaled,
carbon
monoxide
molecules
bond
strongly
to
the
hemoglobin
molecules
in
blood
and
interfere
with
the
transport
of
oxygen
throughout
your
body.
The
article
above
mentions
that
the
CO
poisoning
victims
were
put inside a hyperbaric (high pressure) chamber filled with pure
oxygen. This must force oxygen into the blood and displace
the carbon monoxide.
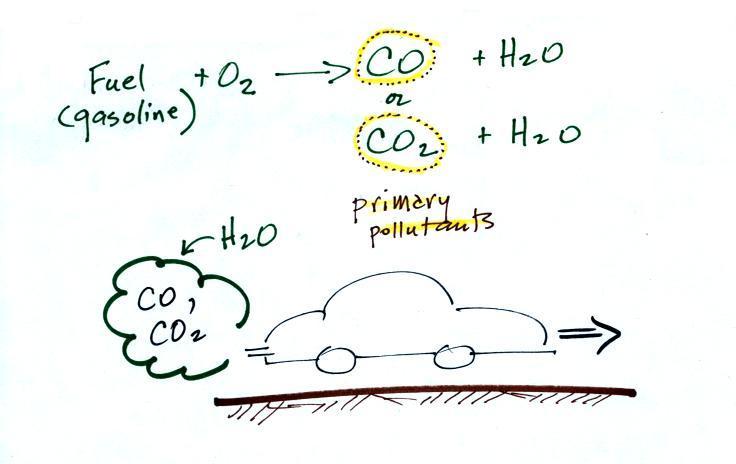
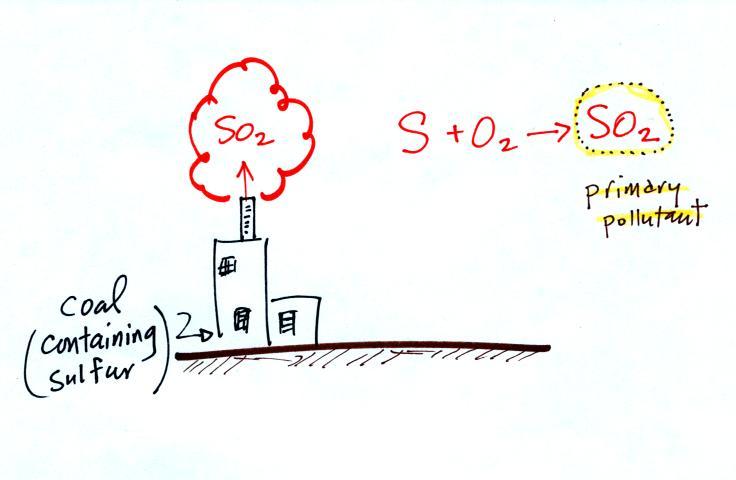
CO is a primary pollutant (Point
2 above). That means it goes directly from a source
into the air, CO is emitted directly from an
automobile tailpipe into the atmosphere for example.
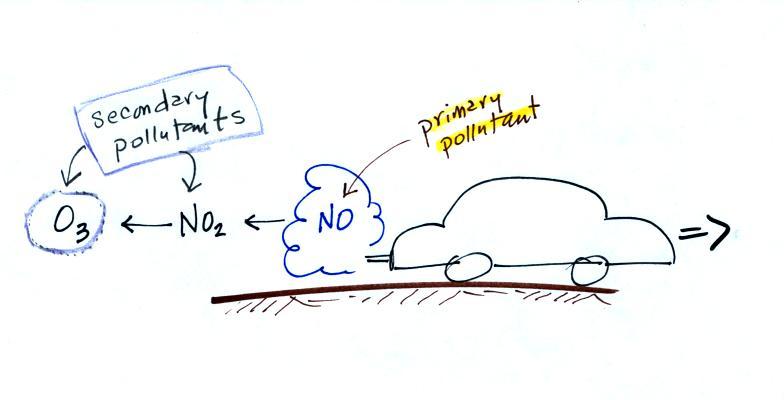
The difference between primary and secondary pollutants
is probably explained best in a series of pictures.
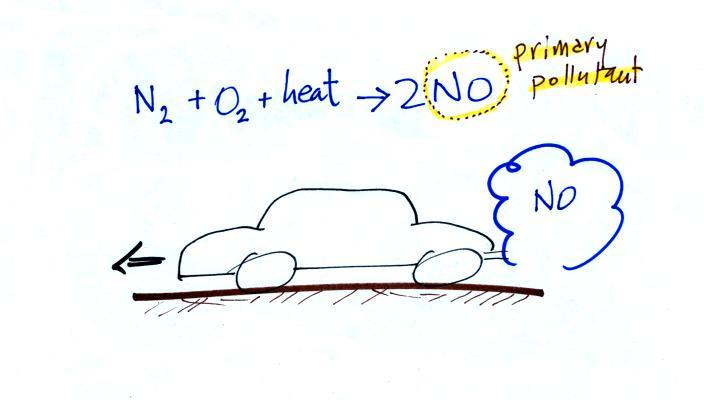
In addition to carbon monoxide, nitric oxide (NO) and sulfur
dioxide (SO2), are also
primary pollutants. They all go directly from a source
(automobile tailpipe or factory chimney) into the
atmosphere. Ozone is a secondary pollutant (and here we mean
tropospheric ozone, not stratospheric ozone). It doesn't
come directly from an automobile tailpipe. It shows up in
the atmosphere only after a primary pollutant has undergone a
series of reactions.
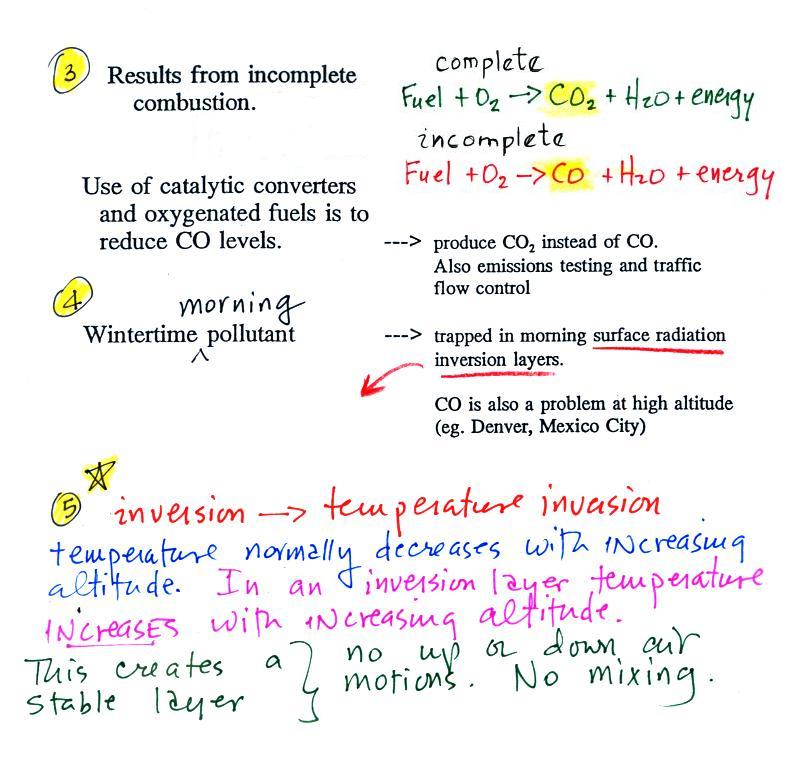
Point 3 explains that CO is produced by incomplete
combustion of fossil fuel (insufficient oxygen). Complete
combustion would produce carbon dioxide, CO2. Cars and trucks
produce much of the CO in the atmosphere in Tucson.
Vehicles must now be fitted with a catalytic converter
that will change CO into CO2
(and also NO into N2 and O2 and hydrocarbons into H2O and CO2).
In Pima County vehicles must also pass an emissions test every
year and special formulations of gasoline (oxygenated fuels) are
used during the winter months to try to reduce CO emissions.
In the atmosphere CO concentrations peak on winter mornings (Point 4). The reason for
this is surface radiation inversion layers. They are most
likely to form on cold winter mornings.
In an inversion layer (Point 5)
air temperature actually increases with increasing altitude which
is just the opposite of what we are used to. This produces
stable atmospheric conditions which means there is little up or
down air motion.
There is very little vertical mixing in a stable air
layer.

In the left figure above, notice how
temperature increases from 40 F to 50 F in the thin air layer
next to the ground (it then decreases with altitude above
that). This is the stable inversion layer. When CO
is emitted into the thin stable layer, the CO remains in the
layer and doesn't mix with cleaner air above. CO
concentrations build.
In the afternoon, the ground warms, and the atmosphere
becomes more unstable. Temperatures decrease with
increasing altitude in the right figure above. CO
emitted into air at the surface mixes with cleaner air
above. The CO concentrations are effectively diluted.

Thunderstorms contain strong up (updraft) and down (downdraft)
air motions. Thunderstorms are a sure indication of unstable atmospheric
conditions.
We'll come back to carbon monoxide on Wednesday.
We'll spend the remainder of the class learning about the
scattering of light. You are able to see a lot of
things in the atmosphere (clouds, fog, haze, even the blue sky)
because of scattering of light. I'm going to try to make a
cloud of smog in class next week. The individual droplets
making up the smog cloud are too small to be seen by the naked
eye. But you will be able to see that they're there because
the droplets scatter light. So we took some time for a
demonstration that tried to show you exactly what light scattering
is.
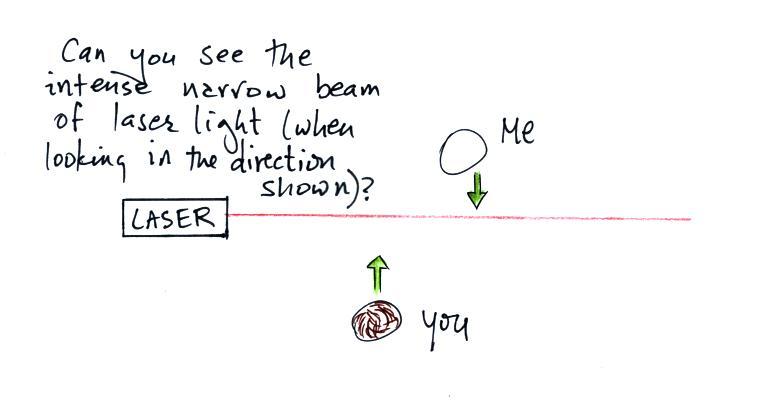
In the first part of the demonstration a narrow beam of intense
red laser light was directed from one side of the classroom to the
other.
Looking down on the situation in the figure above.
Neither the students or the instructor could see the beam of
light. Nobody could see the beam because there weren't any
rays of light pointing from the laser beam toward the students or
toward the instructor.
The instructor would have been
able to see the beam if he had stood at the end of the beam of
laser light and looked back along the beam of light toward the
laser. That wouldn't have been a smart thing to do,
though, because the beam was strong enough to possibly damage
his eyes (there's a warning on the side of the laser).
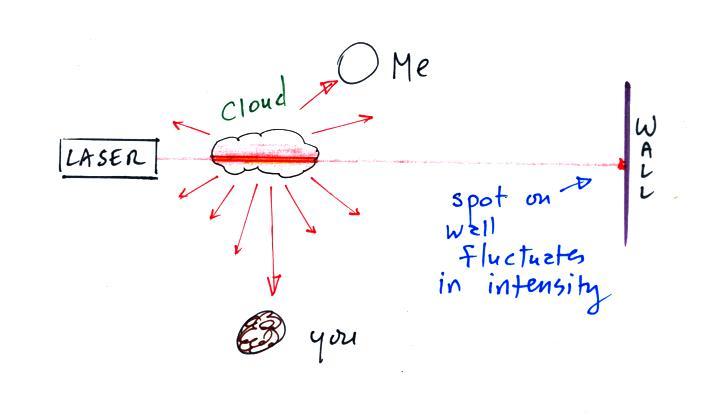
Everybody was able to see a bright red spot where the laser
beam struck the wall.

This is because when the
intense beam of laser light hits the wall it is scattered (splattered is a
more descriptive term). The original beam is broken up
into a myriad of weaker rays of light that are sent out in all
directions. There is a ray of light sent in the
direction of every student in the class. They see the
light because they are looking back in the direction the ray
came from. It is safe to look at this light
because the original intense beam is split up into many much
weaker beams.
Next we clapped some erasers together so that some small
particles of chalk dust fell into the laser beam.

Now instead of a single spot on the wall, students saws lots of
points of light coming from different positions along a straight
segment of the laser beam. Each of these points of light was
a particle of chalk, and each piece of chalk dust was intercepting
laser light and sending light out in all directions. Each
student saw a ray of light coming from each of the chalk
particles.
We use chalk because it is white, it will scatter rather than
absorb visible light. What would you have seen if black
particles of soot had been dropped into the laser beam?
In the last part of the demonstration we made a cloud by pouring
some liquid nitrogen into a cup of water. The cloud droplets
are much smaller than the chalk particles but are much more
numerous. They make very good scatterers.
The beam of laser light really
lit up as it passed through the small patches of cloud.
The cloud droplets did a very good job of scattering laser
light. So much light was scattered that the spot on the
wall fluctuated in intensity (the spot dimmed when lots of light
was being scattered, and brightened when not as much light was
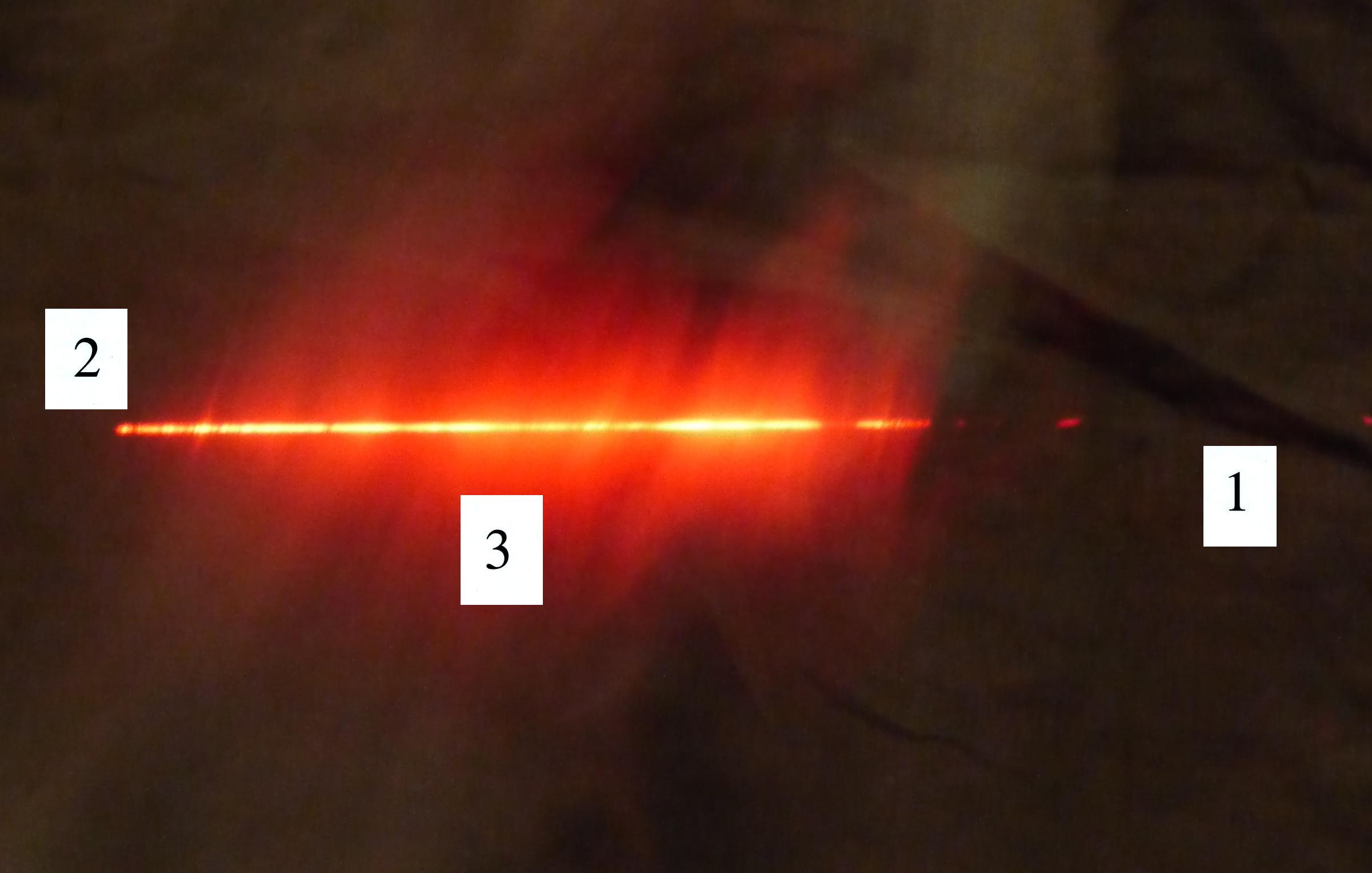
scattered). Here's a photo I took back in my office.
The laser beam is visible in the left 2/3 rds of the picture
because it is passing through cloud and light is being scattered
toward the camera. There wasn't any cloud on the right 1/3rd
of the picture so you can't see the laser beam over near Point 1.
A day or two ago I mentioned that air was invisible.
Well that's not entirely true. Even something as small as
air molecules can scatter light. If you shine a strong
enough light source through enough air you can see the scattered
light.
You can find all kinds of things in the sky: air,
particulates, clouds, etc. But first let's
imagine there isn't an atmosphere. No air, clouds,
particulates, nothing.
If you went outside and looked at the sun (you
shouldn't do that of course) you'd see a bright sun against a
black background. You'd see the sun because you're
looking back in the direction of one of the rays of light
coming from the sun.
If you look away from the sun and toward the sky you
wouldn't see anything. The sky would appear
black. That's because there's nothing to scatter the
sunlight. This is just like when you couldn't see
the laser beam as it traveled across the front of the
classroom. You couldn't see the beam unless
something was put into the beam to scatter some of the
laser light.
In
the
next
picture
we'll
add an atmosphere. Just air molecules, no particles,
or clouds.
Air molecules scatter light. We didn't see this
light in the laser demonstration because the laser light
scattered by the air was too weak. But when you're
dealing with intense sunlight traveling through a lot more
air in the atmosphere you can see the scattered light.
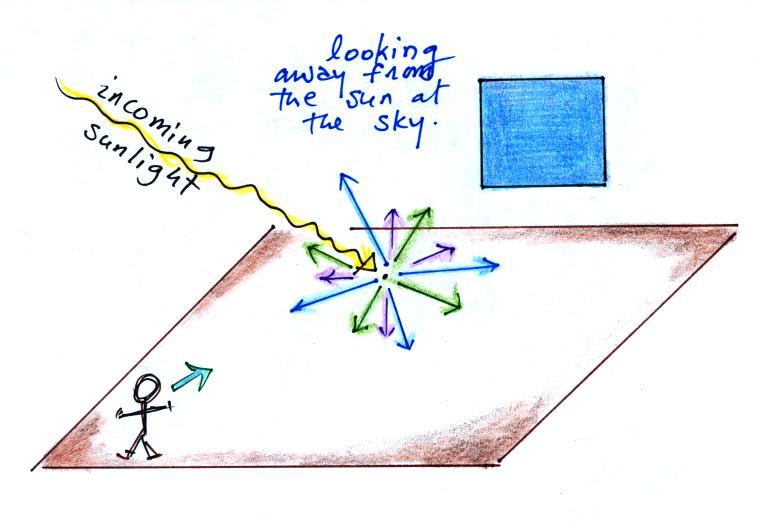
The incoming sunlight is white. White light is a
mixture of all the colors. Air molecules scatter the
shorter wavelengths (violet blue green) more than the
longer wavelengths (yellow orange red). This is
depicted above. Air molecules scatter light in this
way because they are very small (much smaller than the
wavelength of visible light).
Violet has the shortest wavelength and is scattered the
most. However there isn't as much violet in sunlight
as there is blue and green. There's a lot of green
light in sunlight (more than any other color as a matter
of fact) but it isn't scattered as readily as blue.
So the end result is that we see blue light coming from
the sky. This is why the sky is blue. When the
air is clean (from of particulates), the sky has a deep
blue color. Here's
a
little more explanation of why the sky appears blue.
Next we'll add a cloud to the picture. As we saw in
the laser demonstration, cloud droplets and ice crystals
are good scatters of light. Cloud droplets and ice
crystals though are much larger than air molecules.
Because of this they scatter all the colors in equal
amounts.
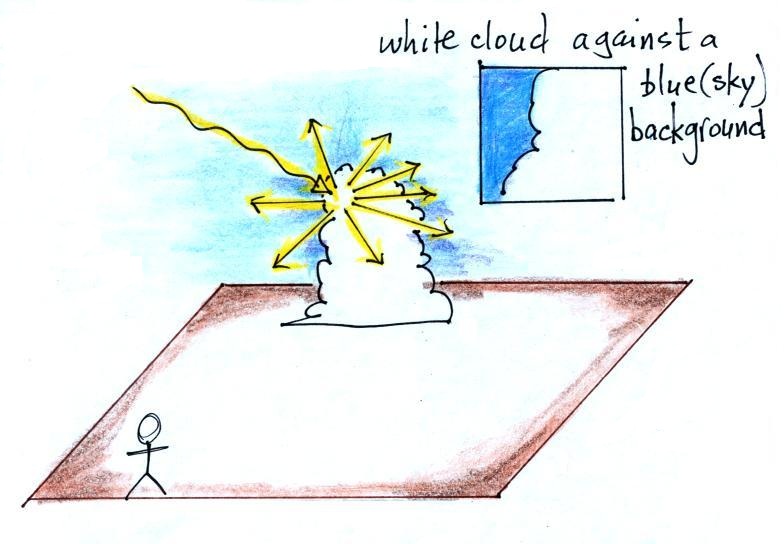
When white light strikes a cloud, white light is scattered
and reflected. This is why clouds are white (with
some shades of grey mixed in if the cloud is thick).
When you look up at a cloud you see a white cloud
(sunlight being scattered by cloud droplets) surrounded by
blue sky (sunlight being scattered by air molecules.
What about particles? Particulates are much bigger
than air molecules and a little bit smaller than cloud
droplets. They scatter light is the same way that
cloud droplets and ice crystals do. The scattered
light from particles is white.
What do you see now when you look at
the sky? It depends on how much particulate matter
is in the air. When the air is clean and doesn't
contain much particulate matter the sky is a deep
blue. As the concentration of particulates increases
you mix in more and more white light. The color of
the sky can change to a whitish blue when the particulate
concentration is high.