Fri., Apr. 4, 2014
Had a little trouble deciding on some music this morning.
I heard The Zombies version of "She's Not
There" on TV in a commercial. Then I stumbled up Santana's
version of the same song. I've been wanting to play
something by Santana for a long time, so I played that. The
Beatles' "While
My Guitar Gently Weeps" is one of my favorite songs of all
time. That made me think of a group I discovered last fall,
Girl in a
Coma and their version of the song.
I want to encourage people to begin preparing for Quiz #3 next
week well before coming to the Monday and Tuesday afternoon
reviews. So something new this time around. To gain
admission to either of the reviews you need to come with evidence
that you have begun to study for the quiz. It can be
something written down or something on a computer but it needs to
be something I can see and evaluate.
The last big topic we will cover
before next week's quiz is precipitation formation and types of
precipitation. Only two of the 10 main cloud types
(nimbostratus and cumulonimbus) are able to produce significant
amounts of precipitation. Why is that? Why is it so
hard for clouds to make precipitation?
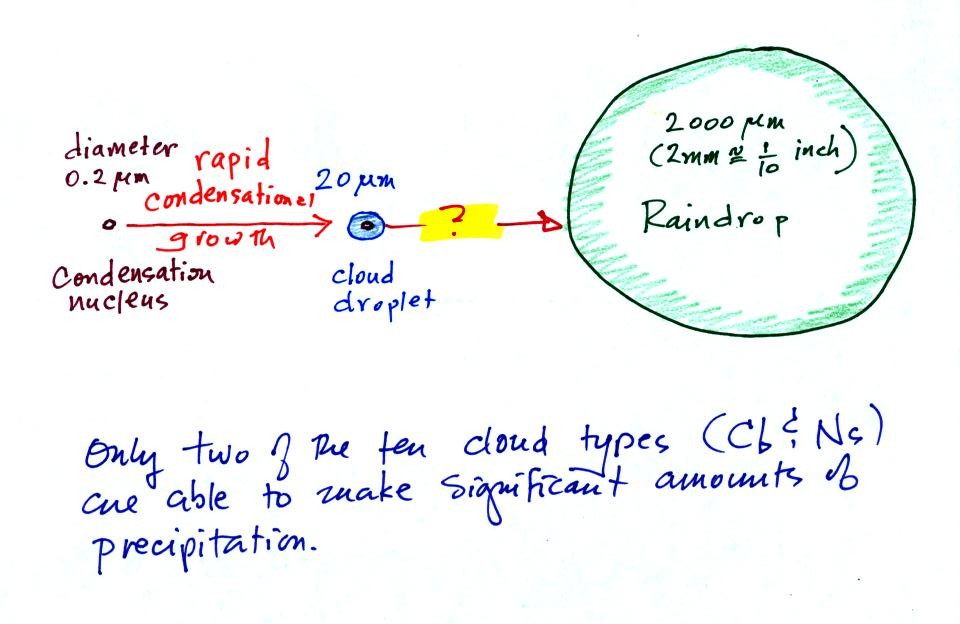
This figure shows typical sizes of cloud condensation nuclei
(CCN), cloud droplets, and raindrops (a human hair is about 50 μm
thick for comparison). As we saw in the cloud in a bottle
demonstration it is relatively easy to make cloud
droplets. You cool moist air to the dew point and raise
the RH to 100%. Water vapor condenses pretty much
instantaneously onto a cloud condensation nucleus to form a
cloud droplet. It would take much longer (a day or more)
for condensation to turn a cloud droplet into a raindrop.
You know from personal experience that once a cloud forms you
don't have to wait days for precipitation to begin to fall.
Part of the problem is that it
takes quite a few 20 μm diameter cloud
droplets to make one 2000 μm diameter
raindrop. How many exactly? Before answering that
question we will look at a cube (rather than a sphere).
How many sugar cubes would you need to make a box that is 4
sugar cubes on a side?
It would take 16 sugar cubes to make each layer and there are 4
layers. So you'd need 64 sugar cubes. Volume is
length x width x height.
The raindrop is 100 times wider, 100 times bigger from front to
back, and 100 times taller than the cloud droplet. The
raindrop has a volume that is 100 x 100 x 100 = 1,000,000 (one
million) times larger than the volume of the cloud droplets.
It takes about a million cloud droplets to make one average
size raindrop.
Fortunately
there
are
two
processes
capable
of
quickly
turning
small
cloud
droplets
into
much
larger
precipitation particles in a cloud.
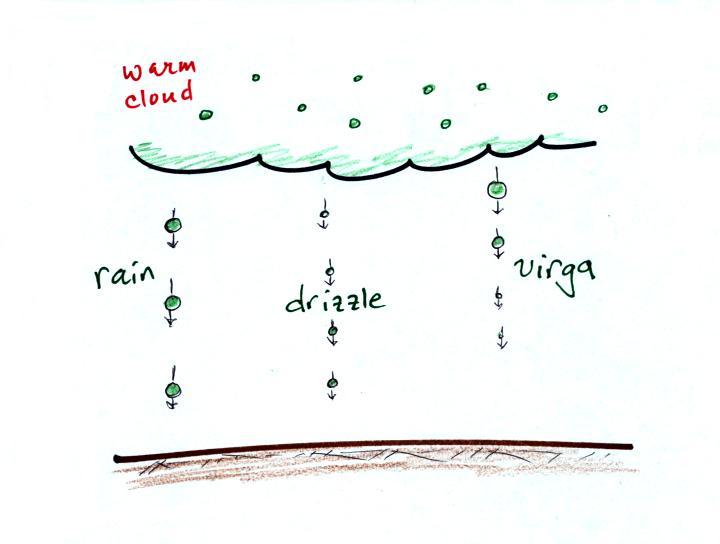
The collision coalescence process works in clouds that
are composed of water droplets only. Clouds like this are
only found in the tropics. We'll see that this is a pretty
easy process to understand.
This process will only produce rain, drizzle, and something
called virga (rain that evaporates before reaching the ground).
The ice crystal process produces precipitation everywhere
else. This is the process that makes rain in Tucson, even on
the hottest day in the summer (summer thunderstorm clouds are tall
and reach into cold parts of the atmosphere, well below
freezing). Hail and graupel often fall from these storms;
proof that the precipitation started out as an ice
particle). Thunderstorms also produce lightning and we will
find that ice is needed to make the electrical charge that leads
to lightning.
There is one part of this process that is a little harder to
understand, but look at the variety of different kinds
of precipitation particles (rain, snow, hail, sleet, graupel, etc)
that can result.
Here's how the collision coalescence process works. The
picture below shows what you might see if you looked
inside a warm cloud with just water droplets:
The collision coalescence process works in a cloud filled with cloud droplets of different
sizes. The larger droplets fall faster than the
small droplets. A larger-than-average cloud droplet will
overtake and collide with smaller slower moving ones.
This is an accelerating growth
process. The falling droplet gets wider, falls faster, and
sweeps out an increasingly larger volume inside the cloud.
The bigger the droplet gets the faster it starts to grow (think
of a growing ball of snow as it rolls down a snow-covered hill
and picks up snow, grows, and starts to roll faster and
faster; or think of an avalanche
that gets bigger and moves faster as it travels downslope)
A larger than average cloud droplet can very
quickly grow to raindrop size.
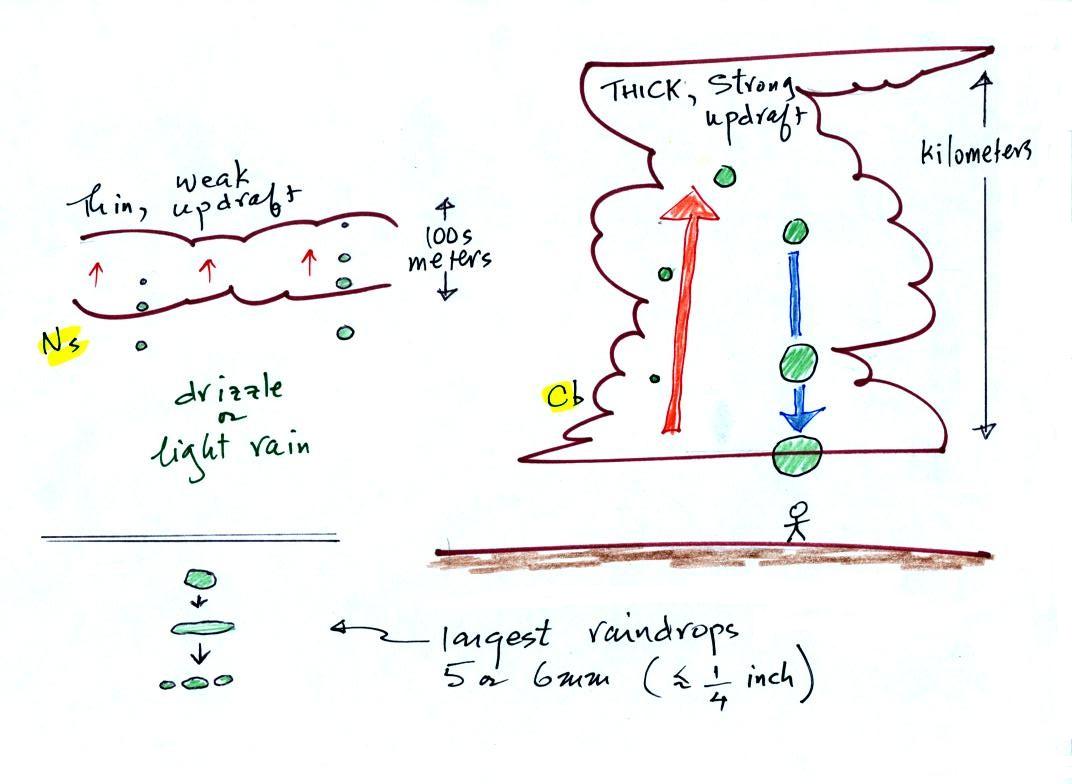
The figure shows the two precipitation producing clouds:
nimbostratus (Ns) and cumulonimbus (Cb). Ns
clouds are thinner and have weaker updrafts than Cb clouds.
The largest raindrops fall from Cb clouds because the droplets
spend more time in the cloud growing. In a Cb cloud raindrops can
grow while being carried upward by the updraft and also when
falling in the downdraft.
Raindrops grow up to about 1/4 inch in diameter. When
drops get larger than that, wind resistance flattens out the drop
as it falls toward the ground. The drop begins to "flop" or
"wobble" around and breaks apart into several smaller
droplets. Solid precipitation particles such as hail can get
much larger (an inch or two or three in diameter).
And actually my sketch at lower left above isn't quite accurate
as this video of the breakup of a
5 mm diameter drop of water shows.
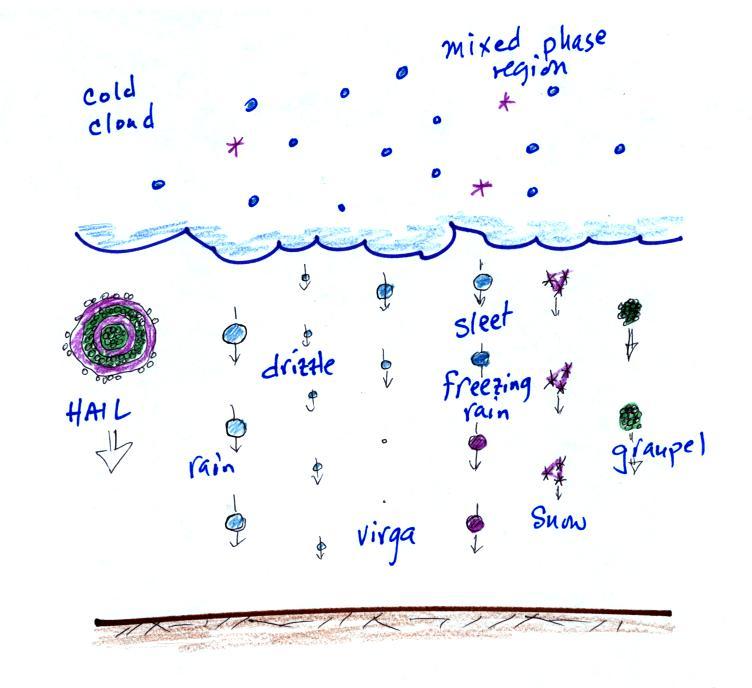
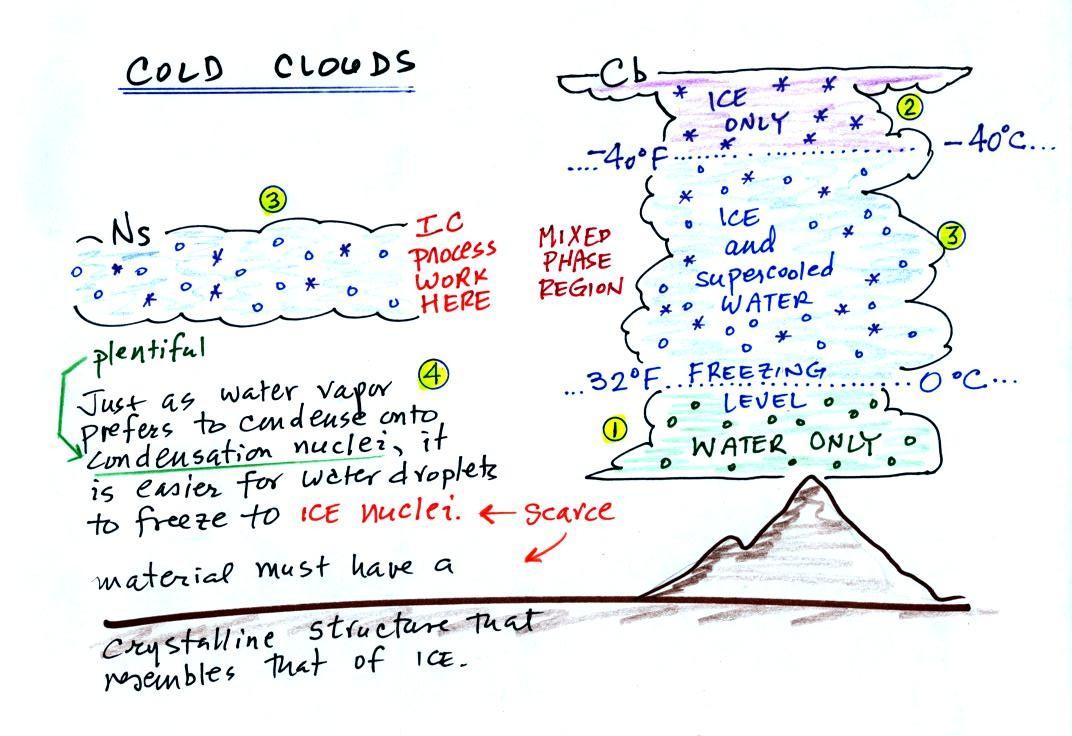
The figure below shows the internal structure of cold clouds.
The bottom of the thunderstorm, Point 1, is warm enough (warmer
than freezing) to just contain water droplets. The top of
the thunderstorm, Point 2, is colder than -40 F (which,
coincidentally, is equal to -40 C) and just contains ice
crystals. The interesting part of the thunderstorm and the
nimbostratus cloud is the middle part, Point 3, that contains both
supercooled water droplets (water that has been cooled to below
freezing but hasn't frozen) and ice crystals. This is called
the mixed phase region.
This is where the ice crystal process will be able to produce
precipitation. This is also where the electrical charge that
results in lightning is created.
The supercooled water droplets aren't able to freeze even
though they have been cooled below freezing. At Point 4 we
see this is because it is much easier for small droplets of water
to freeze onto an ice crystal nucleus or for water vapor to be
deposited onto an ice crystal nucleus (just like it is easier for
water vapor to condense onto condensation nuclei rather than
condensing and forming a small droplet of pure water). Not
just any material will work as an ice nucleus however. The
material must have a crystalline structure that is like that of
ice. There just aren't very many materials with this
property and as a result ice crystal nuclei are rather
scarce. In most of the mixed phase region there are more
water droplets than ice crystals.
Supercooled water
Here are a couple of demonstrations involving supercooled water
that I showed in class. In the first
demonstration, some supercooled water (cooled to -6 F (-21
C)) is poured into a glass bowl sitting at room temperature.
Just pouring the water into the bowl is enough of a "disturbance"
to cause the supercooled water to freeze. Just bumping a
bottle of supercooled water in the second
video is enough to cause the water to freeze. I don't
know why that happens.
Superheated water
It is also possible to superheat water. When the
superheated water is disturbed it suddenly and explosively
boils. This is
potentially dangerous demonstration to attempt, better to watch a video online.
I got the impression after the 2 pm class that there might be one
or two people in the class that were going to try the superheated
water experiment. So here are a couple of precautions.
It is probably easier to superheat distilled water than ordinary
tap water. So you might put two cups of water into a
microwave, one with tap water the other filled with distilled
water. The cup of tap water will probably start boiling when
it is supposed to, i.e. before it can become superheated.
You can watch the tap water and get an idea of how long you need
to heat the distilled water to superheat it.
Then once you think you have superheated the cup of distilled
water be very careful taking it out of the microwave (better yet
leave it in the microwave). Just the slightest disturbance
might start the water boiling. You want your hands, arm,
body and faced covered and protected just in case this happens.
There was a question in the 2 pm class about the
Bubbles in beer or soda
These drinks all contain dissolved carbon dioxide. The
drink containers are pressurized. When you open the can or
take the cap off the pressure inside is released and dissolved
carbon dioxide gas starts to come out of solution and forms small
bubbles. Often you will see the bubbles originate at a point
on the side or bottom of the glass. These are nucleation
sites and are often small scratches or defects on the surface of
the glass that are filled with a small bubble of air. When
the carbon dioxide comes out of solution rather than forming a
bubble of its own, it takes advantage of these existing bubbles of
air. The bubble, now a mixture of air and carbon dioxide,
grows until it is able to break free and float to the surface (a
little gas is left behind so the process can start over again).
The next time you are drinking one of these carbonated beverages
sprinkle in a few grains of sugar or salt. These will serve
as additional nucleation site and additional bubbles will
form.