Mon., Feb. 24, 2014
A couple of songs from Brandi Carlile before class today:
"Helplessly
Hoping" and "The Story".
The 1S1P Assignment #1 reports on stratospheric ozone have been
graded and were returned today. That leaves just the carbon
dioxide topic left to grade. The first of the Assignment #2
topics, the Surface Weather Map Analysis, is due Wednesday this
week.
The Upper Level Charts Optional Assignment was collected today
(that's the one that will earn you a Green Card if you do a good
job on the assignment). The Optional Assignment on the
station model notation and surface weather maps (collected last
Monday) was returned today together with the In-class Optional
Assignment from last Friday. Several students did have a
look at the online lecture notes over the weekend (something
everyone should be doing), found the assignment, and turned it in
at the start of class today. They'll receive credit for the
assignment.
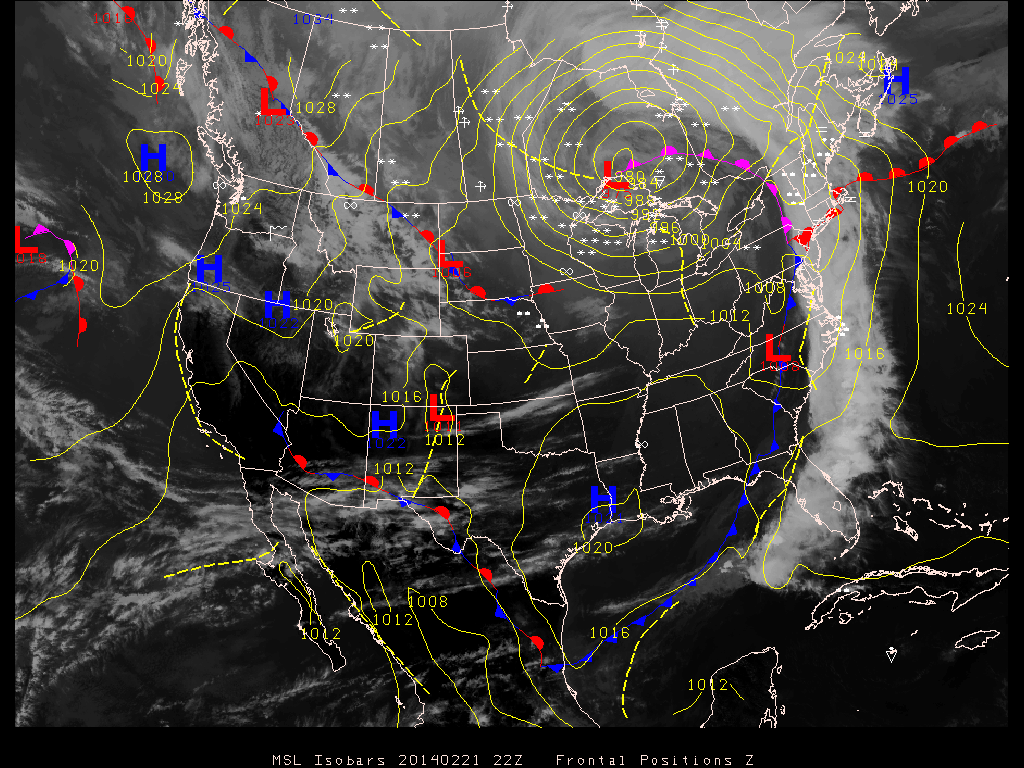
Here's a particularly photogenic storm
system with a long cold front. Pressure at the center of
the storm (up near the Great Lakes) is about 980 mb.
Note the long line of cloudy weather stretching along the
length of the cold front.
You may notice a couple of types of fronts that are
unfamiliar. First extending out from the low pressure
center, in magenta, with both half circles and "points", is an
occluded front.
Occluded fronts form when a cold front, which typically
moves at 15 to 25 MPH, overtakes a warm front, which move at
10 to 15 MPH. This is shown in the sketch below.
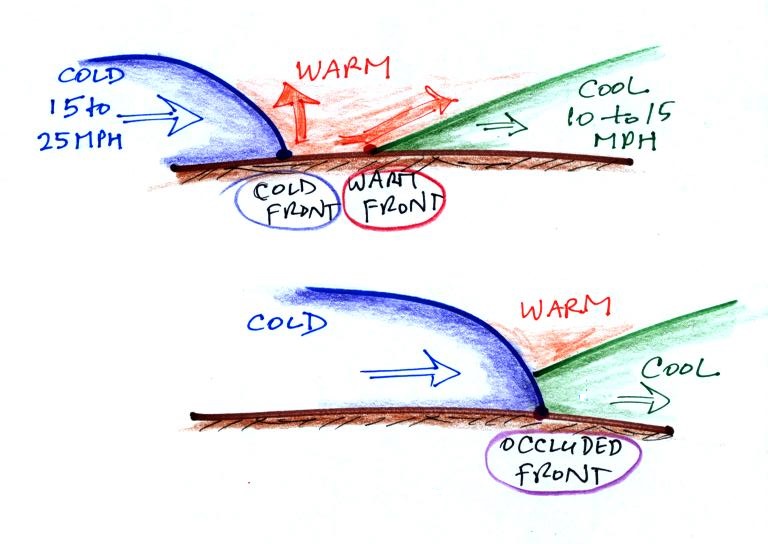
The top figure shows crossectional
views of a cold front and a warm front. The
advancing cold air mass has a rounded shape. At a
warm front warm air overtakes and overuns the back
retreating edge of a cold air mass. The back edge
has a ramp like shape.
When the advancing edge of cold air (the cold front)
catches the retreating edge of cool air at the warm front,
the cold air wedges its way underneath the slightly less
dense cool air. There is no longer any warm at
ground level - it has all been lifted by the colder
air. Because air is being lifted cloud formation and
precipitation is found along occluded fronts.
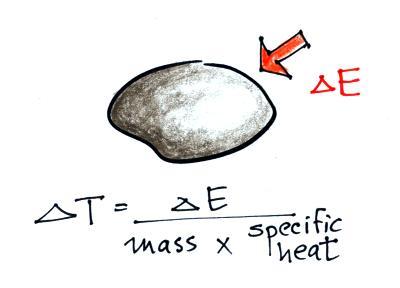
You add energy to something and its
temperature usually increases. The figure below (p.
46 in the ClassNotes) shows you what happens inside an
object when it's temperature changes. (a picture from a
previous semester).

The atoms or molecules inside the warmer object will be
moving more rapidly (they'll be moving freely in a gas, just
"jiggling" around while still bonded to each other in a
solid). Temperature provides a measure of the average kinetic
energy of the atoms or molecules in a material.
You need to be careful what temperature scale you use when
using temperature as a measure of average kinetic energy.
You must use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero kinetic energy. There is no such
thing as negative kinetic energy.
You can think of heat as being the total kinetic
energy of all the molecules or atoms in a material.
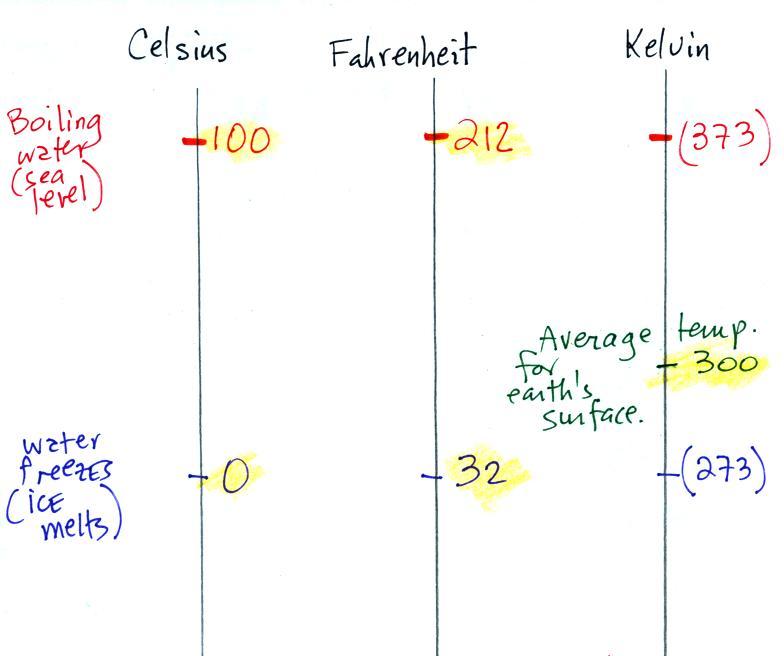
Speaking of temperature scales
You should remember the temperatures of the boiling point and
freezing point of water on at least the Fahrenheit and
Celsius scales (and the Kelvin scale if you want to). 300 K
is a good easy-to-remember value for the global annual average
surface temperature of the earth. Remember 300 K and also
that temperature never goes below zero on the Kelvin scale.

You certainly don't need to try to remember all these
numbers. The world high temperature record value of 136 F
above was measured in Libya at a location that was only about 35
miles from the Mediterranean coast. Water, as we have seen,
moderates climate so it seemed odd that such a high temperature
would have been recorded there. The World Meteorological
Organization recently decided the 136 F reading was invalid and
the new world record is the 134 F measurement made in Death
Valley.
The continental US cold temperature record of -70 F was set in
Montana and the -80 F value in Alaska. The world record -129
F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high
latitude, high altitude, and location in the middle of land rather
than being near or surrounded by ocean (again water moderates
climate, both hot and cold).
Liquid nitrogen is very cold but it is still quite a bit warmer
than absolute zero. Liquid helium gets within a few degrees
of absolute zero, but it's expensive and there's only a limited
amount of helium available. So I would feel guilty bringing
some to class and I don't think it would look any different than
liquid nitrogen.
This next figure might make
clearer the difference between temperature (average kinetic
energy) and heat (total kinetic energy). This figure
(p. 46a in the ClassNotes) wasn't shown in class.
A cup of water and a pool of
water both have the same temperature. The average kinetic
energy of the water molecules in the pool and in the cup are the
same. There are a lot more molecules in the pool than in
the cup. So if you add together all the kinetic energies
of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the
molecules in the cup. There is a lot more stored energy in
the pool than in the cup. It would be a lot harder to
change the total energy of the water in the pool, i.e. cool (or
warm) all the water in the pool, than it would be to change the
total energy of the water in the cup.
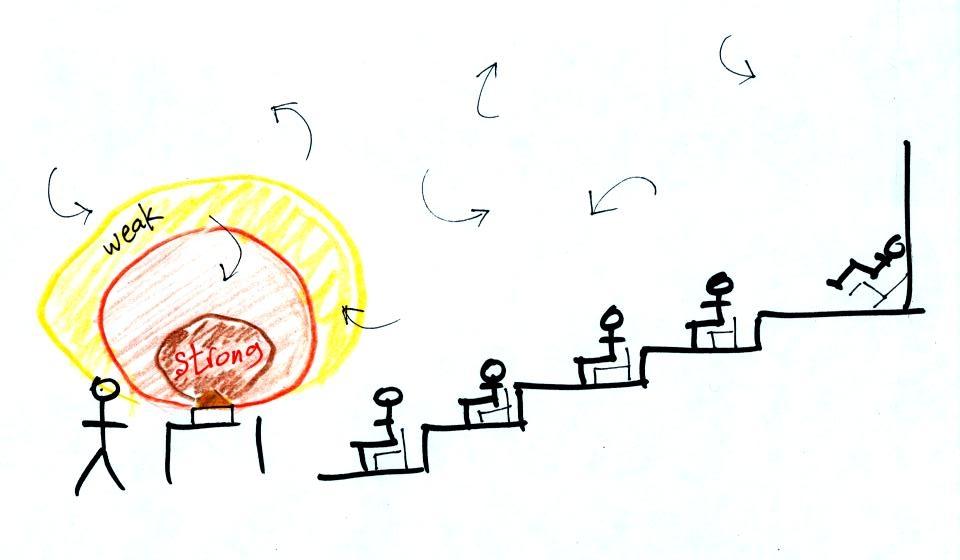
The difference between
temperature and heat can be understood by considering groups of
people and money (the people represent atoms or molecules and
the money is analogous to kinetic energy). Both groups
above have the same $10 average amount of money per person
(that's analogous to temperature). The $100 held by the
larger group at the left is greater than the $20 total possessed
by the smaller group of people on the right (total amount of
money is analogous to heat).
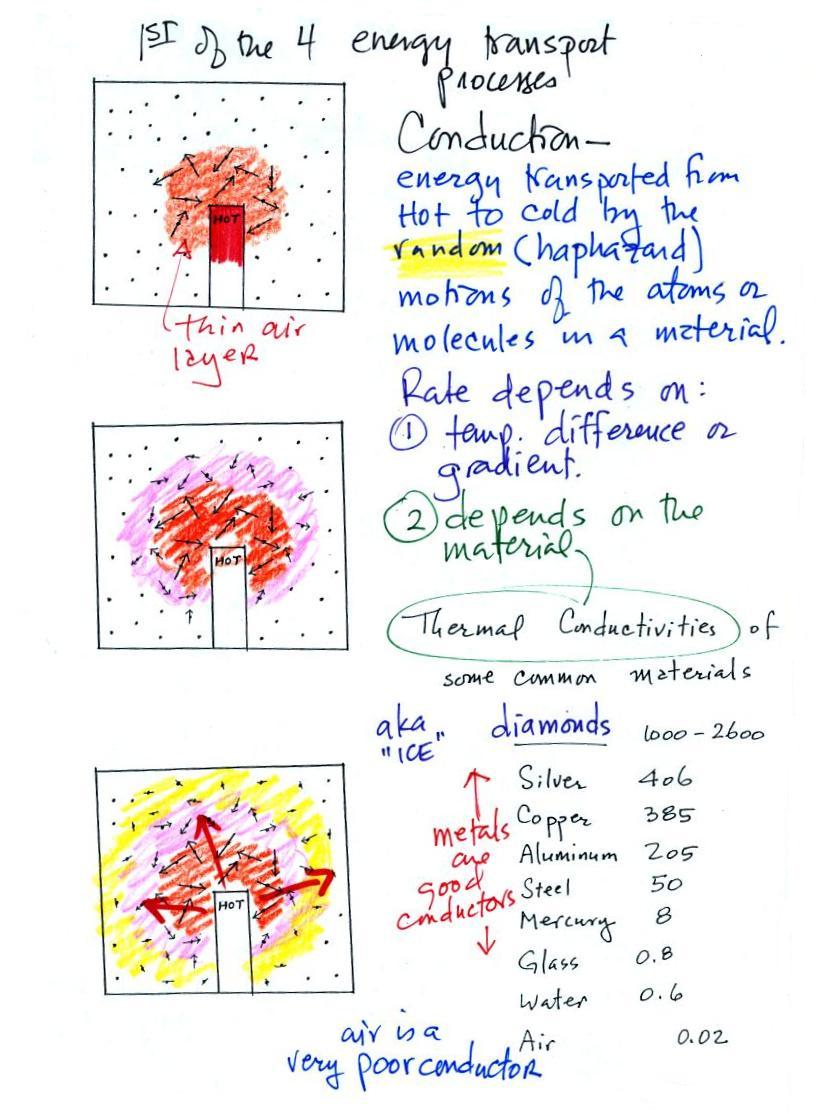
Energy transport by conduction
Conduction is the first of four energy transport processes that we
will cover (and the least important transport process in the
atmosphere). The figure below illustrates this
process. Imagine heating the end of a piece of copper tubing
just so you can visualize a hot object. If you held the
object in air it would slowly lose energy by conduction and cool
off.
How does that happen? In the top picture some of the
atoms or molecules near the hot object have collided with the
object and picked up energy from the object. This is
reflected by the increased speed of motion or increased kinetic
energy of these molecules or atoms (these guys are colored
orange).
In the middle picture the initial layer of energetic molecules
have collided with some of their neighbors and shared energy with
them (these are pink). The neighbor molecules have gained
energy though they don't have as much energy as the molecules next
to the hot object.
In the third picture molecules further out (yellow) have now
gained some energy. The random motions and collisions
between molecules is carrying energy from the hot object out into
the colder surrounding air.
Conduction transports energy from hot to cold. The
rate
of
energy
transport
depends
first
on
the
temperature
gradient
or
temperature
difference
between
the
hot object and the cooler surroundings. If the object in the
picture had been warm rather than hot, less energy would flow and
energy would flow at a slower into the surrounding air.
The rate of energy transport also depends on the material
transporting energy (air in the example above). Thermal
conductivities of some common materials are listed. Air is a
very poor conductor of energy and is generally regarded as an
insulator.
Water is a little bit better conductor. Metals are generally
very good conductors (cooking pans are often made of stainless
steel but have aluminum or copper bottoms to evenly spread out
heat when placed on a stove). Diamond has a very high
thermal conductivity (apparently the highest of all known
solids). Diamonds are sometimes called "ice." They
feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
I brought a propane torch to class to demonstrate the behavior
of materials with different thermal conductivities. Because
of time constraints I only actually demonstrated the rightmost
picture in class. I'll have the torch again in class on
Wednesday and will demonstrate how much better a conductor copper
is than glass.
A piece of copper tubing is held in the flame in the picture at
left. Copper is a good conductor. Energy is
transported from the flame by the copper and you must grab the
tubing several inches from the end to keep from burning your
fingers. Part of a glass graduated cylinder is held in the
flame in the center picture. You could comfortably hold onto
the cylinder just a couple of inches from the end because glass is
a relatively poor conductor. The end of the glass tubing got
so hot that it began to glow (its is emitting radiant energy, the
4th of the energy transport processes we will discuss). Air
is such a poor conductor that it is safe to hold your finger just
half an inch from the hot flame and still not feel any heat coming
from the flame (but be careful putting your hand or fingers above
the flame)
Transport of energy by conduction is similar to the transport
of a strong smell throughout a classroom by diffusion. Small
eddies of wind in the classroom blow in random directions and move
smells throughout the room. For a demonstration you need
something that has a strong smell but is safe to breathe.
Last semester I had great hopes for Vicks Vapo Rub which
contains Camphor, Eucalyptus Oil and Menthol. But that
didn't work very well. So this semester I again tried curry
powder.
It didn't work very well either. The
classroom is too large and the ventilation system too
efficient. Though sometimes a demonstration that doesn't
really work can be instructive. I'll bring the curry
powder to class again on Wednesday and we'll add a little
change to the demonstration and hopefully get the smell to
spread further out into the room.
Because air has such a low thermal conductivity it is often
used as an insulator. It is important, however, to keep the
air trapped in small pockets or small volumes so that it isn't
able to move and transport energy by convection (we'll look at
energy transport by convection on Wednesday). Here are some
examples of insulators that use air:
Foam is often used as an insulator. Foam is
filled with lots of small air bubbles, they're what provides
the insulation.
You can safely hold onto a foam cup filled with liquid
nitrogen (-320 F) because the foam does such a good job
insulating your fingers from the cold liquid inside.
Thin insulating layer of air in a double pane
window. I
don't have double pane windows in my
house. As a matter of fact I leave a
window open so my cats can get in and out of the
house (that's not particularly energy
efficient).
We really haven't needed winter coats much this winter
in Tucson.
Hollow fibers (Hollofil) filled with air used in
sleeping bags and ski coats.
Goose feathers (goosedown) work in a similar way.
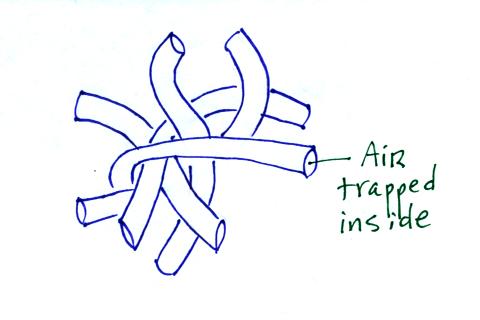
Fiberglas insulation is another example. It works so
well as an insulator first because it is glass which has low
thermal conductivity and also because it traps lots of little
pockets of air.
We really didn't cover much material today because I wanted to
leave time for an in-class experiment. Three
students from class volunteered to help.
Here's the object of the experiment:
The source of energy in our experiment will be the energy
contained in a cup of room temperature water. We'll pour
some liquid nitrogen into the water. The water will cool as
energy is taken from it and used to evaporate liquid nitrogen.
We'll be able to use a thermometer to measure how much the
water cools and use that to determine how much energy was taken
from the water. This is illustrated below:
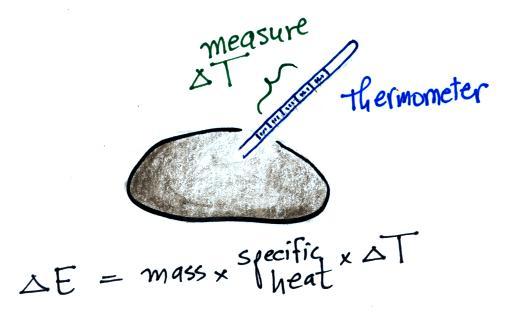
As we saw last Friday, adding energy to an object will cause it to
warm up. If you know how much energy you added, the object's
mass and specific heat, you can calculate the temperature change
that will result using the left equation above.
Now we'll just rearrange the terms in the equation so that we can
use a measurement of temperature change to determine the amount of
energy added or removed. That's the equation at right.
We start with a styrofoam cup filled about 1/3 full with room
temperature water.
The cup and the water together weighed
169.5 g of room temperature water. The cup weighed 4.0 g, so
we really had 165.5 g of water. The students measured its
temperature, 23 C.
Next they poured some liquid nitrogen into a second, smaller
styrofoam cup.
We're going to evaporate 33.0 grams of liquid nitrogen.
The total amount of energy needed to do that, ΔE, is
the mass of the liquid nitrogen times the Latent Heat of
Vaporization of Nitrogen (LHvap).
ΔE = mass x LHvap
LHvap is the energy needed per gram to vaporize
(evaporate) liquid nitrogen. That's the quantity we are
trying to measure.