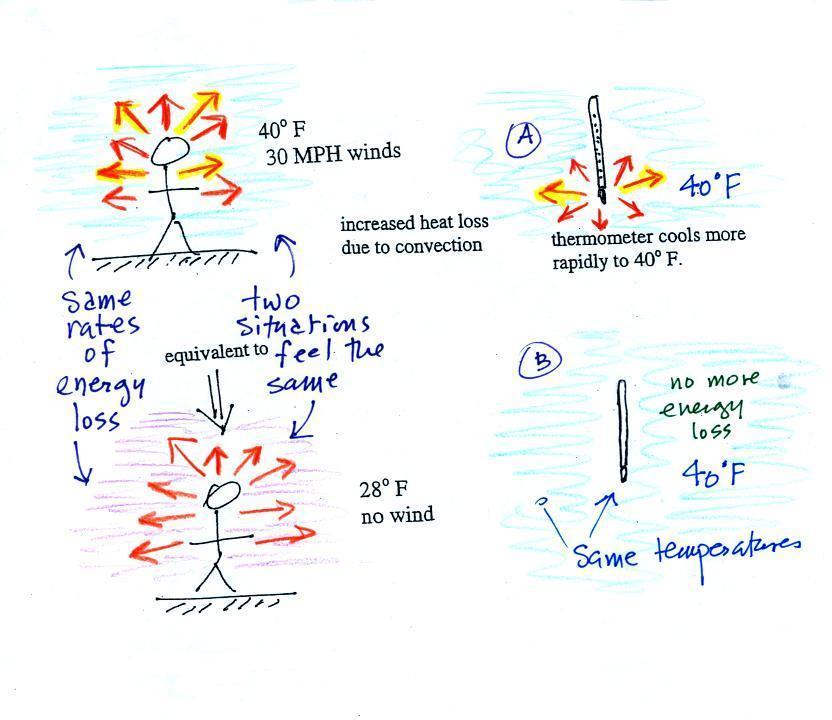
You can consciously remove energy
from water vapor to make it condense. You take
energy out of water to cause it to freeze (you could put
water in a freezer; energy would flow from the
relatively warm water to the colder surroundings).
If one of these phase changes occurs, without you
playing a role, energy will be released into the
surroundings (causing the surroundings to warm).
Note the orange energy arrows have turned around and are
pointing from the material toward the
surroundings. It's kind of like a genie coming out
of a magic lamp. One Tedy Bruschi worth of kinetic
energy is released when enough water freezes to make an
ice cube. Many genies, many Tedy Bruschis, are
released when water vapor condenses.
This release of energy into the surroundings and the
warming of the surroundings is a little harder for us to
appreciate because it never really happens to us in a
way that we can feel. Have you ever
stepped out of an air conditioned building into warm
moist air outdoors and had your glasses or sunglasses
"steam up"? Water vapor never condenses onto your
much too warm body. However if it did you would
feel warm. It would be just the opposite of the
cold feeling when you step out of the shower or a pool
and the water on your body evaporates. You know
how cold the evaporation can make you feel, the same
amount of condensation would produce a lot of warming.

A can of cold drink will warm more
quickly in warm moist surroundings than in warm dry
surroundings. Equal amounts of heat will flow from
the warm air into the cold cans in both cases.
Condensation of water vapor is an additional source of
energy and will warm that can more rapidly. I
suspect that the condensation may actually be the
dominant process.
The foam "cozy", "koozie",
or whatever you want to call it, that you can put around
a can of soda or beer is designed to insulate the can
from the warmer surroundings but also to keep water
vapor in the air from condensing onto the can.
Now two figures to illustrate how latent heat energy
transport works.

1. You've just stepped out of
the shower and are covered with water. The water
is evaporating and energy is being taken from your
body.
2. The water vapor (containing the energy from
your body), is free to move from the bathroom to the
kitchen where a cold can is sitting on a table.
3. Water vapor comes into contact with the cold
can and condenses. The hidden latent heat energy
in the water vapor is released into the can and warms
the drink inside.
Energy has effectively been transported from your
warm body in the bathroom to a cold can in the kitchen.