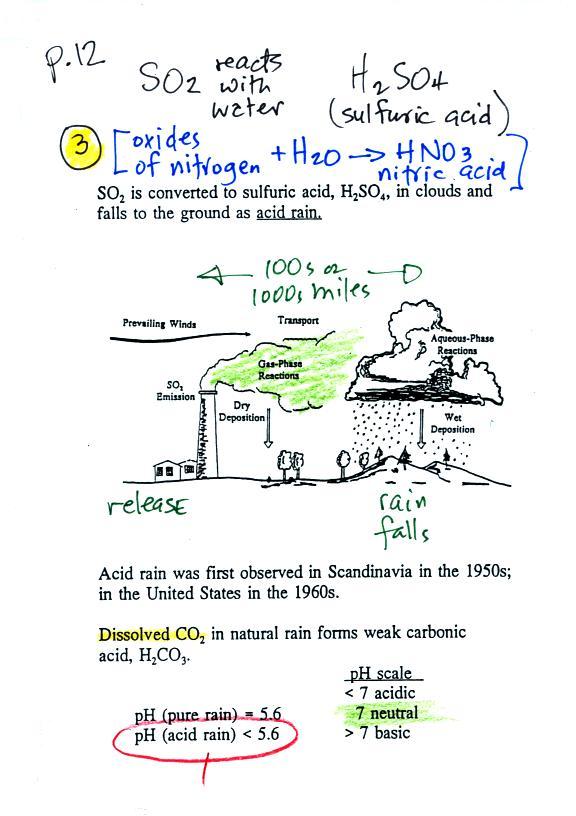

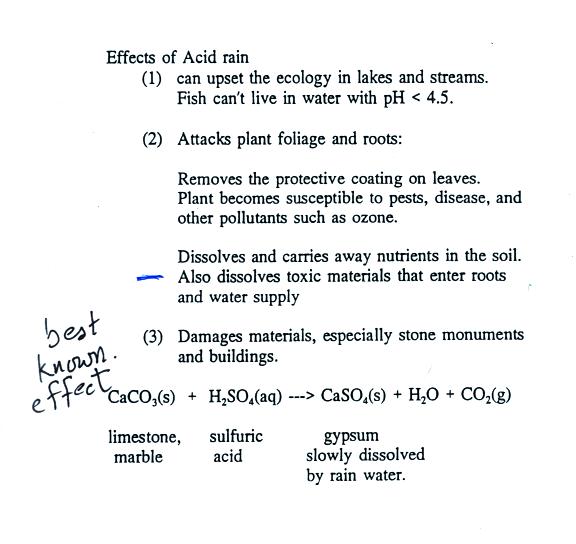

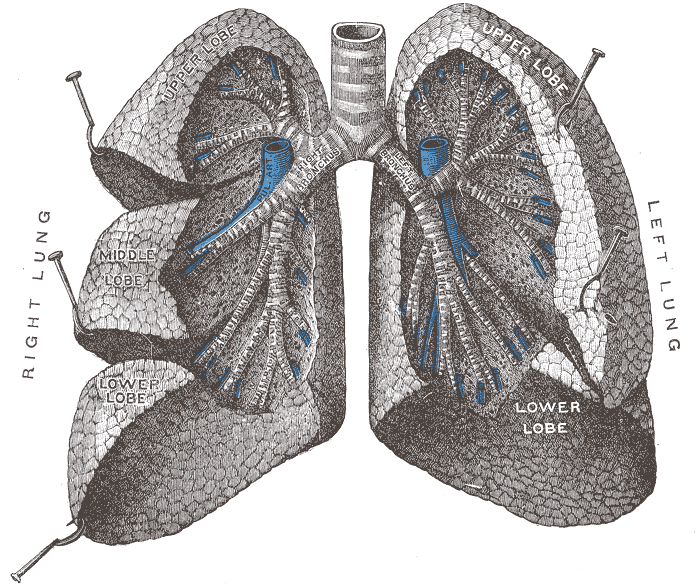
 |
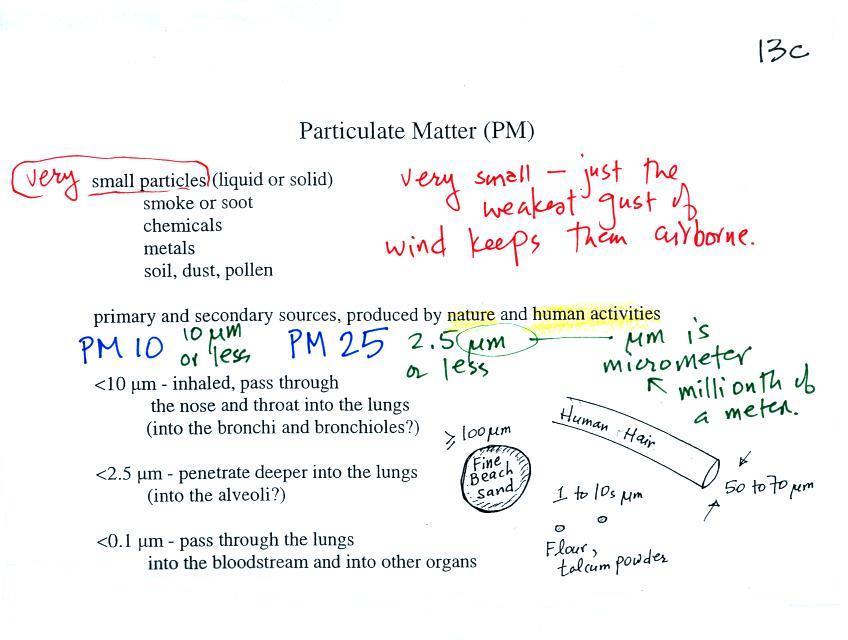
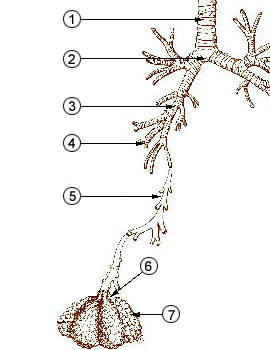 1 - trachea
from http://en.wikipedia.org/wiki/Image:Illu_quiz_lung05.jpg2 - mainstem bronchus 3 - lobar bronchus 4 - segmental
bronchi
5 - bronchiole6 - alveolar duct 7 - alveolus |
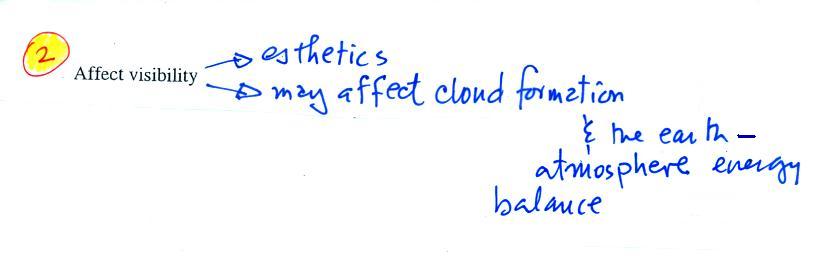



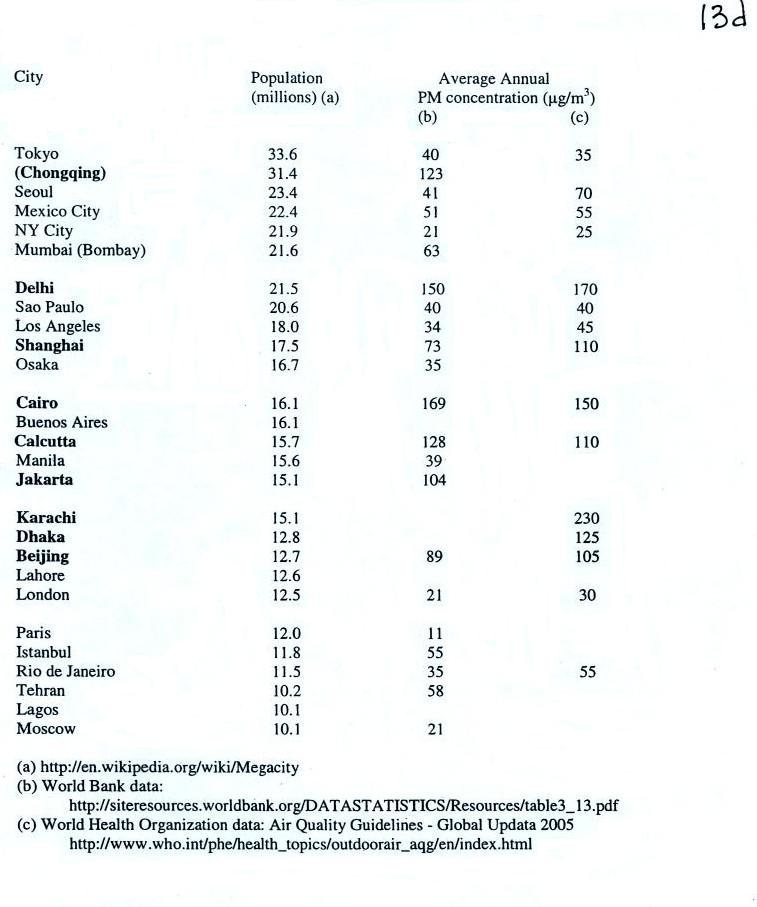

 |







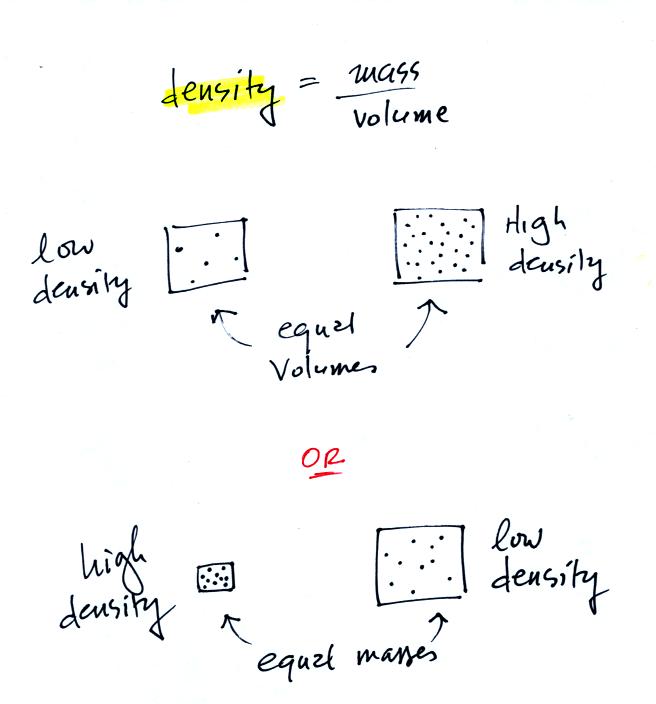
| material |
density g/cc |
| air |
0.001 |
| redwood |
0.45 |
| water |
1.0 |
| iron |
7.9 |
| lead |
11.3 |
| mercury |
13.6 |
| gold |
19.3 |
| platinum |
21.4 |
| iridium |
22.4 |
| osmium |
22.6 |