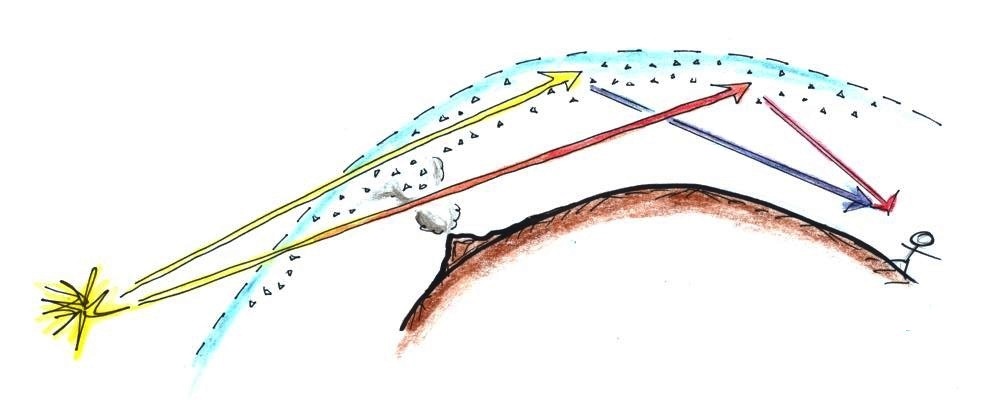
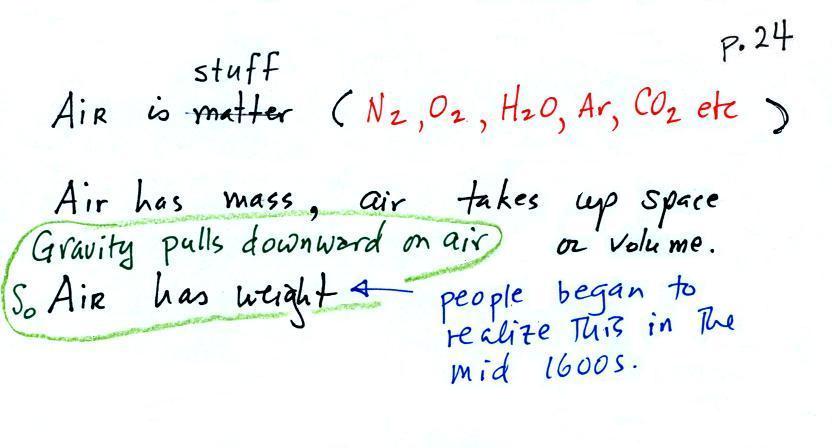
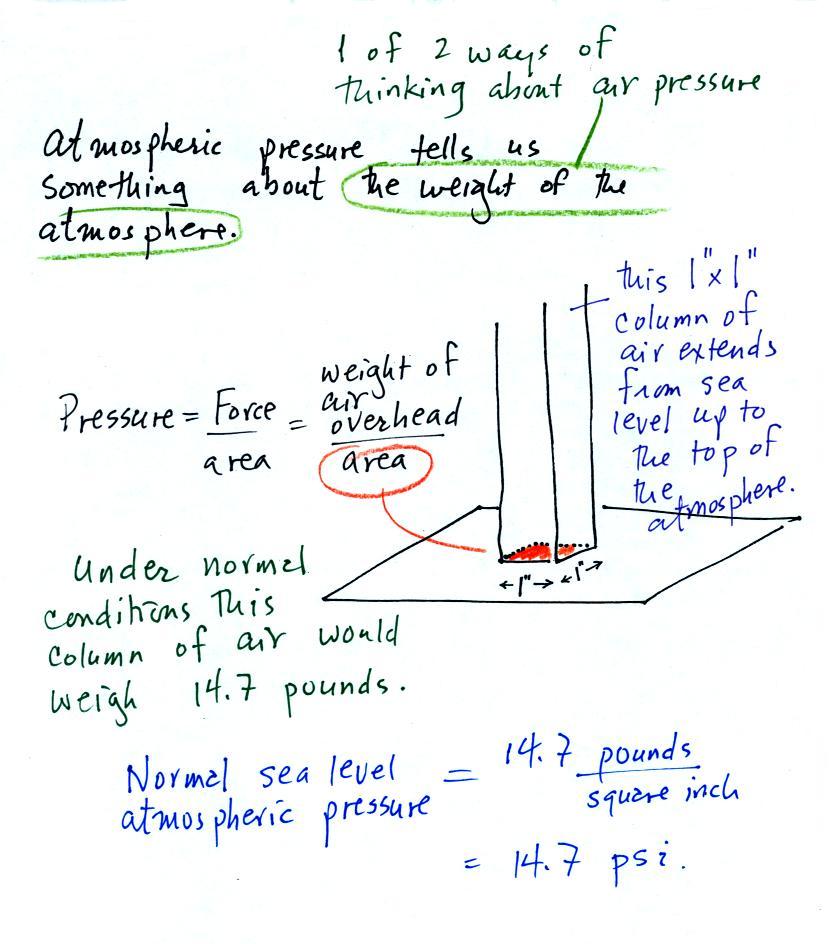
There's a lot going on in this picture, we'll examine
it step by step.
1.The sea level pressure is the same, 1000 mb, in both
pictures. Since pressure is determined by the weight
of the air overhead, the weight of the air overhead in the
left picture is the same as in the right picture.
The amount (mass) of air above sea level in both pictures
is the same.
2. There is a 100 mb drop in pressure in
both air layers. Pressure has decreased because air
that was overhead (the air between the ground the level of
the dotted line) is now underneath. Because the
pressure change is the same in both pictures the weight of
the air layers are the same. The thin layer at left
has the same weight as the thicker layer at right.
Both layers contain the same amount (mass) of air.
3. Both layers contain the same amount (mass) of
air. The air in the layer at left is thinner.
The air is squeezed into a smaller volume. The air
in the layer at left is denser than the air in the layer
at right.
4. To determine the rate of pressure decrease you
divide the pressure change (100 mb for both layers) by the
distance over which that change occurs. The 100 mb
change takes place in a shorter distance in the layer at
left than in the layer at right. The left layer has
the highest rate of pressure decrease with increasing
altitude.
So both the most rapid rate of pressure decrease with
altitude and the densest air are found in Layer A.
The fact that the rate of pressure decrease with
increasing altitude depends on air density is a fairly
subtle but important concept. This concept will come
up 2 or 3 more times later in the semester. For
example, we will need this concept to explain why
hurricanes can intensify and get as strong as they
do.