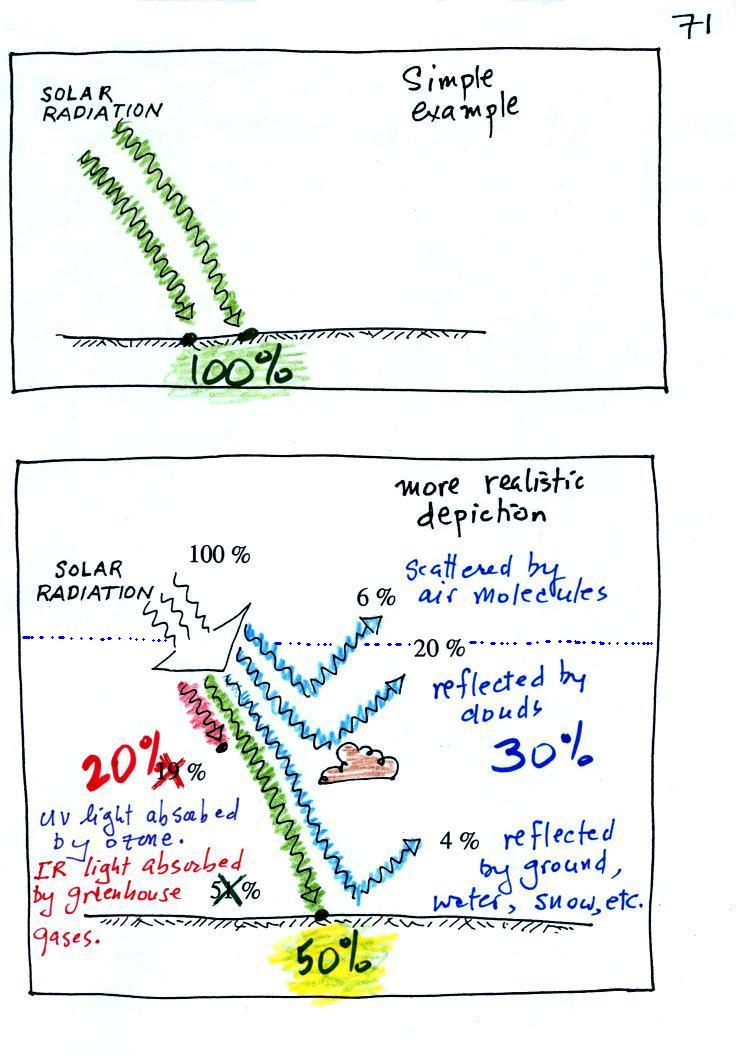
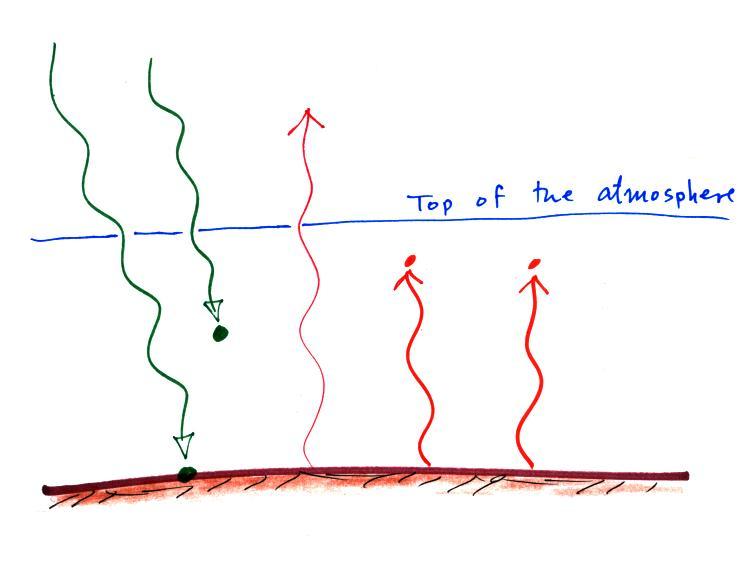
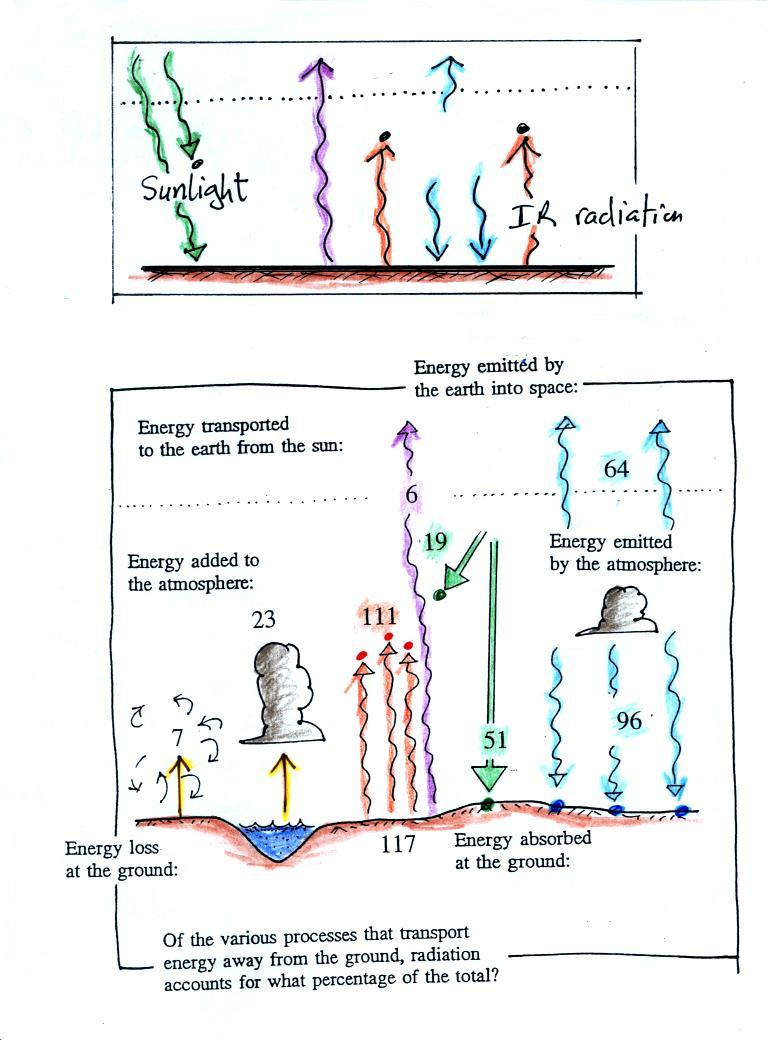
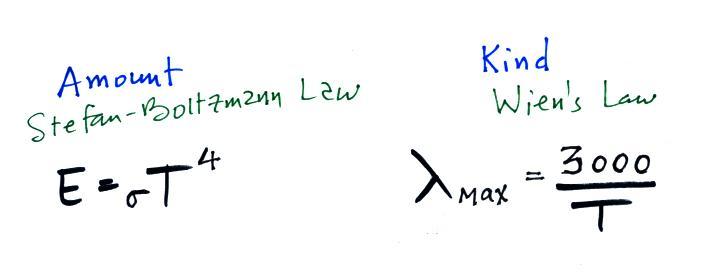
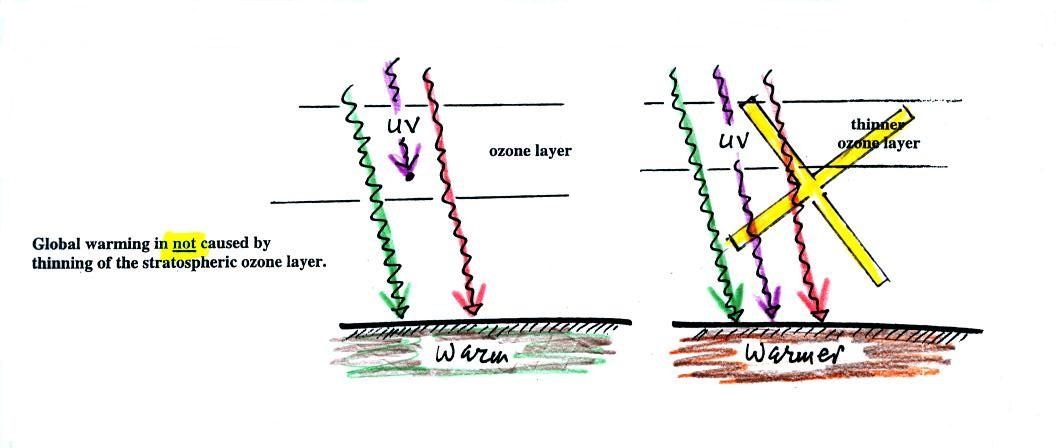
We can use our simplified
representation of the greenhouse effect to understand the
effects of clouds on daytime and nighttime temperatures.
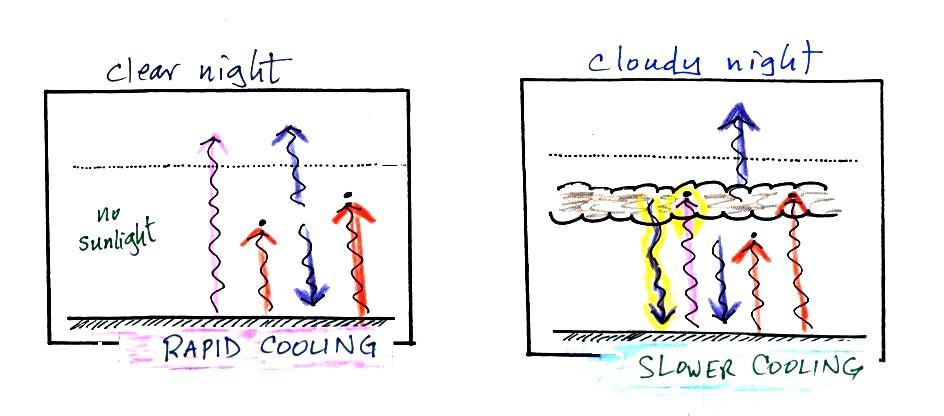
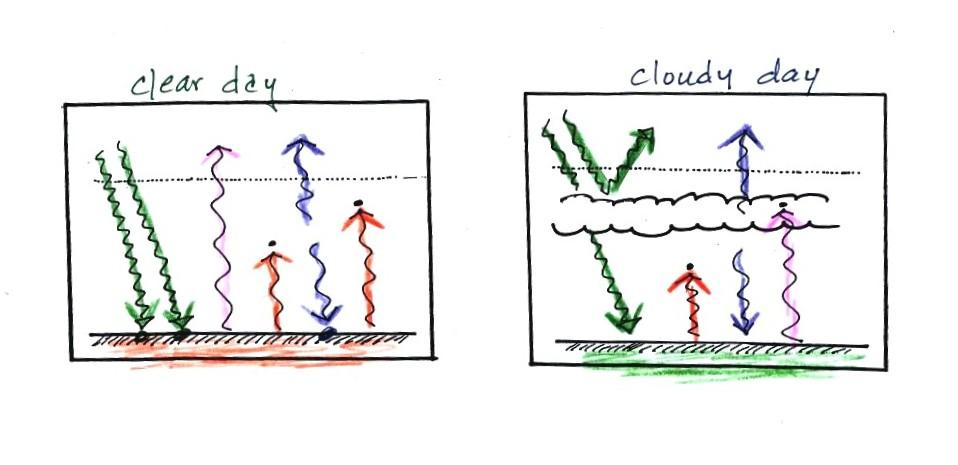
Does it get colder or stay warmer on a cloudy night compared
to a clear night. Does it get hotter or stay cooler on a
cloudy day compared to a clear day. The following can be
found on pps. 72a & 72b in the ClassNotes (I've rearranged
things slightly to try to make it clearer)
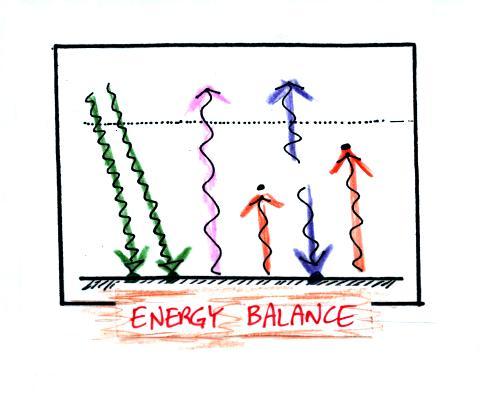
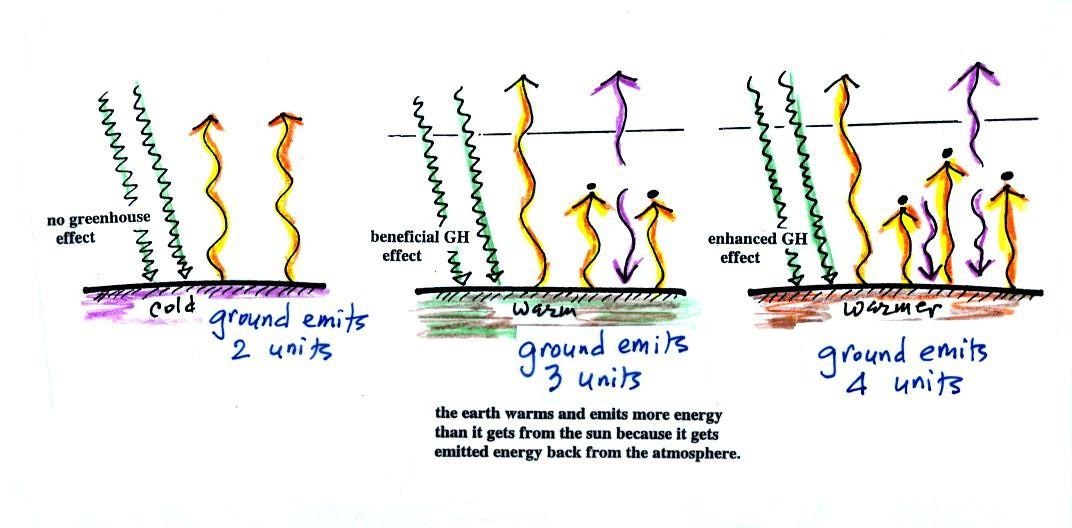
Here's the answer to the question found
near the start of today's notes
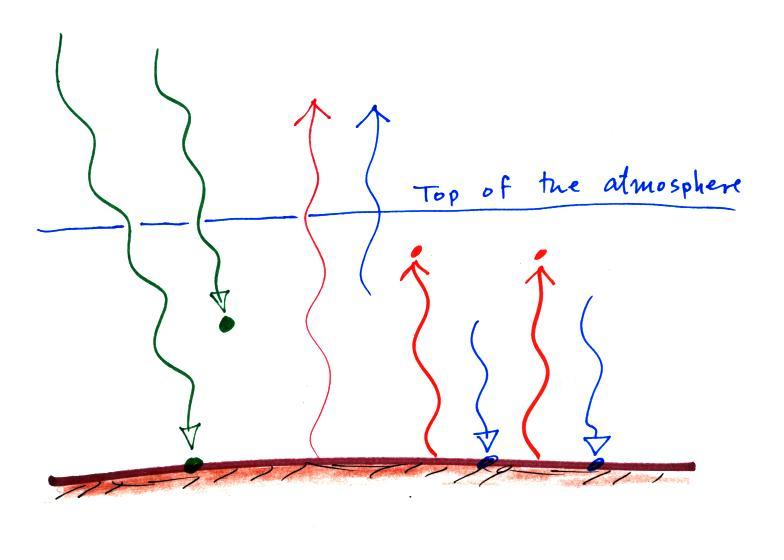
The atmosphere is absorbing three units of
energy: one comes from the sun,
two are IR light coming from the
ground.
To see how those 3 units should be deployed we need to look at
the ground. The ground is absorbing 1 unit of sunlight
and emitting 3 units of IR light. It needs 2 arrows of the IR emitted by the
atmosphere. The 3rd
unit of atmospheric IR goes up and into space.