The next topic concerns how the human body exchanges energy (or heat) with its surroundings. This will include how the body responds to both hot and cold temperatures AND how humidity and winds factor into the heat exchange. This will lead us to the concepts of wind chill factor and heat index.
We start with a few basics. Keep in mind the material presented here is somewhat simplistic. In reality energy expenditures and transfers can do more than just change the temperature of an object.
Radiation is the transmission of energy through space or through a material medium in the form of electromagnetic waves. Don't be concerned about understanding the wording of the last sentence. Only a couple of points will be made now. We will return to radiation later in the semester.
All objects in the universe emit (or give off) radiation energy. The type and amount of radiation energy emitted depends on the object's temperature. Basically, the hotter the object, the greater the amount of radiation energy it emits. For example, the Sun emits much more radiation energy than the Earth because the Sun is much hotter.
If you place a rock out in space, the rock loses energy, and hence cools down, by continuously emitting radiation. Meanwhile, the rock gains energy, and hence heats up, by absorbing radiation energy that was originally emitted by other objects, like stars. If the radiation energy absorbed by the rock is greater than the radiation energy emitted by the rock, the temperature of the rock will go up. If radiation energy absorbed is less than radiation energy emitted, the temperature of the rock will go down.
This explains much of the daily temperature changes at a given place on the Earth. At night, the ground surface cools because it is emitting radiation energy away, while there is no radiation energy coming in from the Sun. During the day, the ground surface heats up because the radiation energy being absorbed from the Sun is greater than the radiation energy being emitted from the ground.
The human body also exchanges radiation with its surroundings. Often the air surrounding us is cooler than the body temperature. This means the body emits more radiation than it receives from the air and there is a net cooling due to radiation. However, if we are standing above a hot ground surface in the middle of the day, the transfer of energy by radiation is from the hotter ground to the body. Exposure to direct sunlight also delivers radiation energy to the body.
Conduction is the transfer of energy by direct collisions of molecules (touching). Energy can be conducted from one object to another or within a single object that contains temperature variations (See Figure F). The rate at which energy is transferred within a material is referred to as its heat conductivity. For example, take a rod of steel. Heat the rod at one end and measure how quickly heat is conducted toward the other end. In general, solids and liquids are better heat conductors than gases because the molecules that make up solids and liquids are more tightly packed than in gases. Thus, water and metals are good heat conductors, while air is a poor heat conductor (or a good heat insulator). A table of heat conductivities for several substances is provided below. You don't need to worry about the scientific units of heat conductivity. Use the table to compare how well heat is transferred by conduction through various materials. The higher the heat conductivity, the faster heat flows through the material by conduction. Note that some of best heat insulators, items at the bottom of the table like wood, rabbit fur, and wool, get much of their insulating properties from trapped air within the material. For example, wool jackets keep us warm mainly because of the pockets of still air trapped within the fibers.
|
Material |
Thermal Conductivity (cal/sec)/(cm2C/cm) |
|---|---|
| Diamond | 2.38 |
| Copper | 0.99 |
| Aluminum | 0.50 |
| Water Ice | 0.0050 |
| Glass | 0.0025 |
| Concrete | 0.0020 |
| Water at 20°C | 0.0014 |
| Dry Sand | 0.0013 |
| Body Tissue, muscle | 0.00092 |
| Body Tissue, fat | 0.00047 |
| Wood | 0.00019 |
| Rabbit Fur | 0.000065 |
| Wool | 0.000061 |
| Still Air at 0°C | 0.000057 |
When two different objects touch heat is always transferred from the warmer object to the colder object. If you touch something hot, energy is transferred from the hot object to you. If you touch something cold, energy is transferred from you to the cold object.
The rate of conductive heat transfer depends on:
Differences in conductivity between water and air also partially explains why swimming in water at a temperature of 70°F (21°C) feels cold, while standing outside when the air temperature is 70°F (21°C) does not. Because water is a good heat conductor, it moves heat away from your body faster than air does, which results in a cold sensation. Another reason different from conduction is that water has a large heat capacity, which means water must absorb a lot of heat (energy) to raise its temperature. So if you are surrounded by a large pool of cold water, the heat from your body is easily conducted away from you and does not cause the water to warm up much, which allows the conductive heat transfer to remain rapid.
Convection is the transfer of heat by actual movement of mass within a fluid. Convection is a very important means of energy transport in the atmosphere, especially moist convection. Convection only occurs in fluids (liquids and gases), not in solids. In the atmosphere, we can think of convection happening when parcels of air (blobs of air about the size of large balloons) move around.
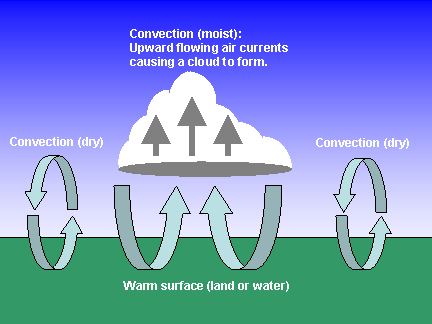
Two types of convection are important in the atmosphere:
All three mechanisms of energy transfer, conduction, convection, and radiation, play a role in how the human body exchanges energy (heat) with the external world. The next page describes how the human body deals with heat and cold stress and how weather conditions impact heat loss from the body.