Acid rain
Sulfur
dioxide is one of the pollutants that can react with
water in clouds to form acid rain (some of the
oxides of nitrogen can also react with water to form
nitric acid). The formation and effects of
acid rain are discussed on p. 12 in the photocopied
Class Notes.
Acid rain is often a problem in regions that
are 100s even 1000s of miles from the source of the sulfur
dioxide. Acid rain in Canada could come from sources in
the US, acid rain in Scandinavia came from industrialized areas
in other parts of Europe.
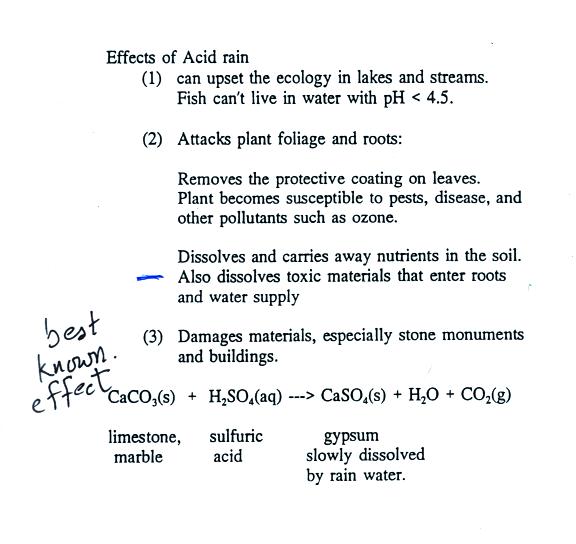
Note at the bottom of the figure above that natural
"pristine" rain has a pH less than 7 and is slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. The acid rain demonstration described below
and done in class should make this point clearer.
Some of the problems associated with acid rain are
listed above.
Acid Rain Demonstration
Some common acids are listed below. In
solution the acid molecules dissociate (split) into pieces.
The presence of H+ ions is what makes these materials
acids.
And
actually it isn't enough to just have H+ ions for
something to be an acid. There are H+
ions in pure distilled water and it's not an acid. To be an
acid the H+ ion concentration must be
greater than is found in distilled water. The H+
ion concentration in distilled water is 10-7 moles
of H+ ions per liter of
water. A mole is
just a number, a very large number (6 x 1023).
It's the same idea as dozen. A dozen means you've got 12 of
something. 10-7 moles
of H+ ions per liter is
10-7 times
6 x 1023 = 6 x 1016
H+ ions per liter of water.
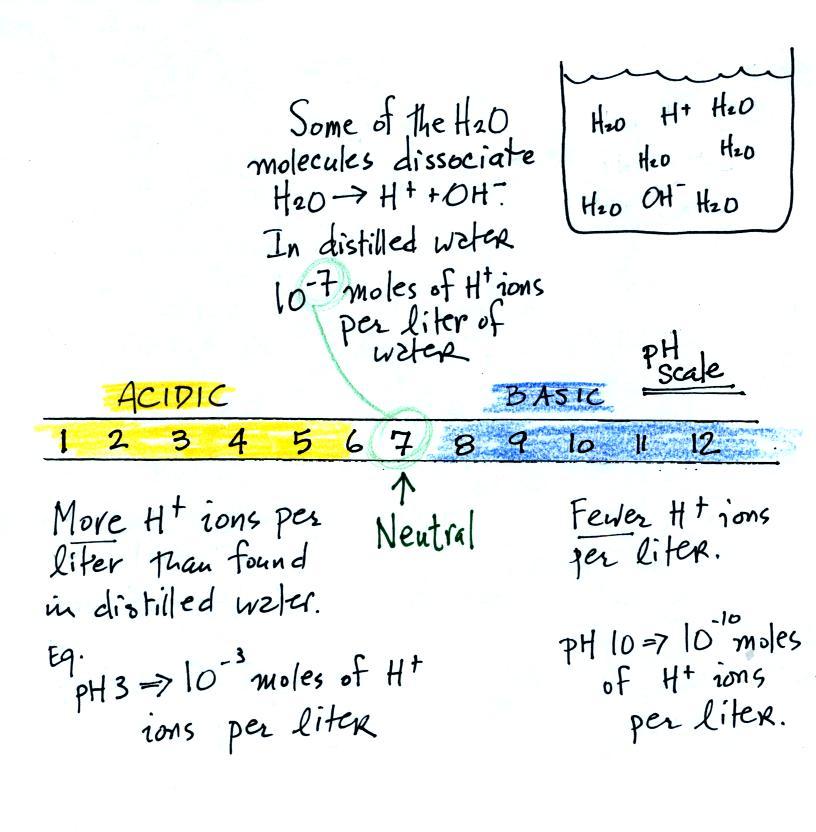
The pH scale
We often use the pH scale to measure acid concentration. An
H+ ion concentration of 10-7 moles/liter corresponds to
pH 7 (the pH value is computed by taking the -log10
of the H+
ion concentration). Other than remembering the pH
value of distilled water is pH7, these are all details
you don't need to worry about.
It is also possible to have fewer H+
ions in a solution than would be found in distilled water. A
solution like this is basic.
Pouring some acid into water would increase the H+ ion concentration (from 10-7moles/liter to 10-3moles/liter, perhaps as shown
in the example above). Adding a base to water will decrease
the H+ ion
concentration (from 10-7moles/liter
to 10-10moles/liter,
perhaps).
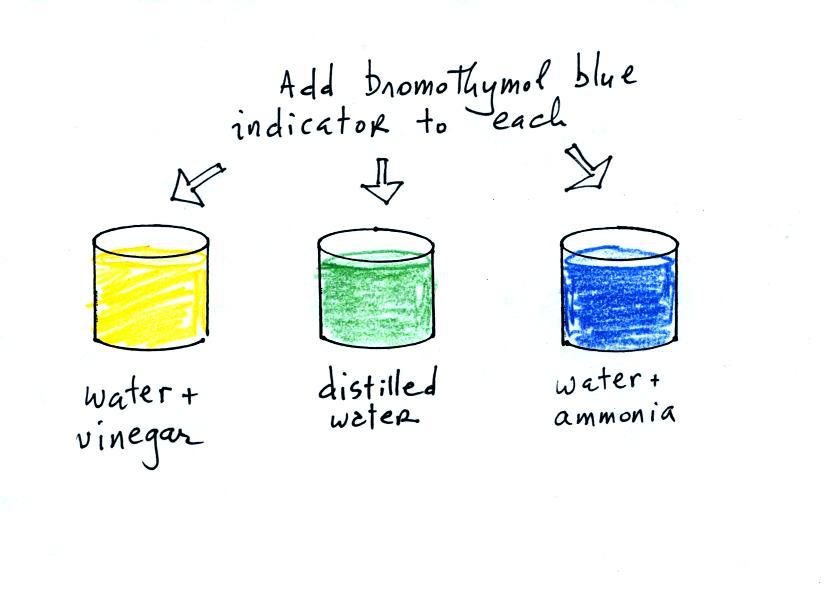
Now we can proceed with the demonstration. We will start
with three 1000 mL beakers each filled with distilled water.
Some vinegar (contains acetic acid) was added to the left beaker.
Some ammonia (a base) was added to the right beaker.
Acid/Base indicator solution
Then we added some bromothymol blue,
a color indicator solution, to all three beakers.
Bromothymol blue has the amazing property of changing color
depending on whether it is mixed with an acid (golden yellow) or a
base (deep blue).
So far we have just reviewed the pH scale and introduced
acid/base indicator solutions.
When sulfur dioxide is released into the air it reacts with the
water in clouds to produce acid rain. I really can't use SO2
in class because it's poisonous. I'll use carbon dioxide, CO2,
instead.
We added some Tucson tap water to a large 2000 mL beaker.
This represents a cloud. We added some bromothymol
blue to the tap water and it turned blue. So we know that
Tucson tap water is basic.
A few small pieces of dry ice are put into a flask. We close
the flask with a stopper. The end of a piece of tubing
connected to the flask is immersed in the tap water.
Dry ice sublimes. It turns directly from solid to ice
(ordinary ice melts and turns from solid to liquid). The
gaseous CO2 is invisible but
you can tell it is there because of the bubbles in the tap
water. Some of the CO2
dissolves as it bubbles through the water and slowly turns the
water acidic. You can tell that this is occurring because
the bromothymol blue indicator turns from deep blue to green and
eventually to yellow.
I call this a "sort of" acid rain demonstration.
That's because we haven't really produced acid rain. Air
contains carbon dioxide and the CO2 makes natural rain slightly
acidic (pH5.6 or so). To make true acid rain we would need a
different gas, something other than carbon dioxide, something that
would lower the pH below 5.6.
While we didn't actually produce acid rain, there is concern
that increasing atmospheric concentrations of carbon dioxide will
dissolve in ocean water and lower the pH of the world's
oceans. This could in turn affect organisms in the ocean
especially those that make shells.
We'll finish by mentioning carbonated beverages which contain
dissolved carbon dioxide and are acidic. Soft drinks also
contain phosphoric acid which makes them even more acidic than the
dissolved carbon dioxide would do. With time the acidity of
soft drinks can damage tooth enamel.