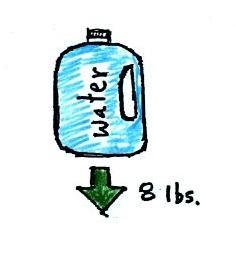Monday, Feb. 5, 2018
Junius Meyvant "Color Decay"
(4:28), "Be
a Man" (3:05), "Pearl in
Sandbox" (6:29), "Gold Laces"
(3:51), "Signals"
(4:10)
Hjalmar "Hljoðlega
af stað" (5:31), "Í
gegnum móðuna" (4:57),
"Hvert
sem ég fer" (3:50)
Ylja "Út"
(3:33)
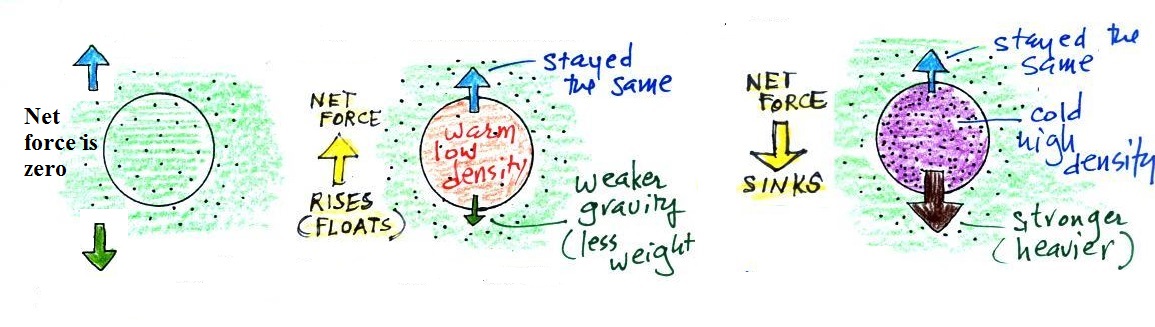
Step #3 Two vertical forces acting on a parcel of air
in the atmosphere
(see p. 53 in the ClassNotes)
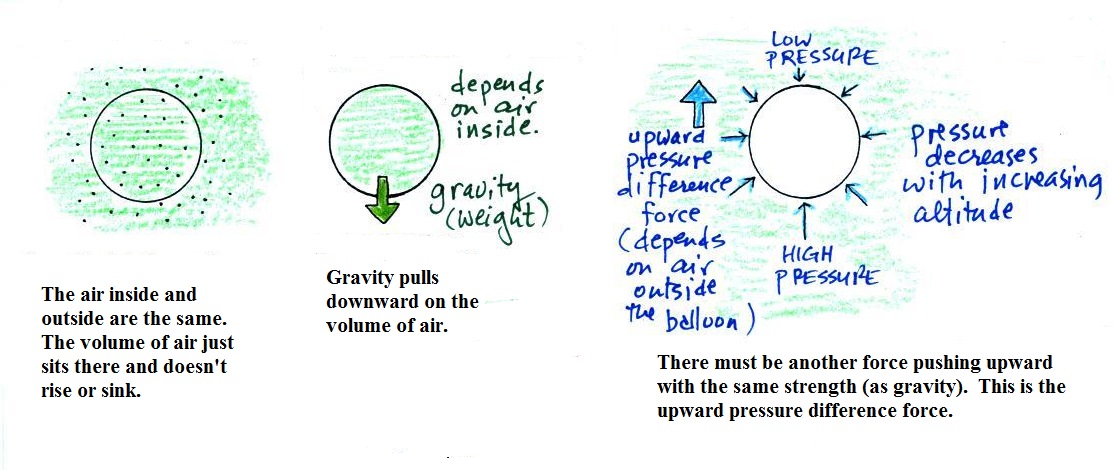
Basically it comes down to this - there are
two forces acting on a parcel of
air in the atmosphere. They are shown above.
The first force is gravity, it pulls downward. Most
everyone knows about this force. The strength of the
gravity force (the weight of the air in the parcel) depends on
the mass of the air inside
the parcel.
Second there is an upward pointing pressure difference
force. Not too many people know about this one. This
force is caused by the air outside (surrounding) the parcel.
Pressure decreases with increasing altitude. The pressure
of the air at the bottom of a parcel pushing upward is slightly
stronger than the pressure of the air at the top of the balloon
that is pushing downward. The overall effect is an upward
pointing force.
When the air inside a parcel is exactly the same as the air
outside (same densities), the two forces are equal in strength
and cancel out. The parcel is neutrally buoyant and it
wouldn't rise or sink, it would just hover.
We'll replace the air inside the balloon with
either warm low density air or cold high density air.
In the first case, a balloon with warm low density air won't
weigh as much. The gravity force is weaker. The
upward pressure difference force doesn't change (because it is
determined by the air outside the balloon which hasn't changed)
and ends up stronger than the gravity force. The balloon
will rise.
Conversely if the air inside is cold high density air, it
weighs more. Gravity is stronger than the upward pressure
difference force and the balloon sinks.
It all comes down to a question of how the density of the air
in a parcel compares to the density of the air surrounding the
parcel. If the parcel is filled with low
density air it will rise. A parcel full of high density
air will sink. That's true of things other than air.
Wood floats in water because it is less dense than water.
Here's a short demonstration of the role
that density plays in determining whether a balloon will rise or
sink (or hover)
Convection demonstration
We used balloons filled with helium (see
bottom of p. 54 in the photocopied Class Notes).
Helium is less dense than air even when it has the same
temperature as the surrounding air. The downward
gravity force (weight of the helium filled balloon) is
weaker than the upward pressure difference force. You
don't need to warm a helium-filled balloon to make it rise.
We dunk the helium filled balloon in
liquid nitrogen to cool it off. When you pull the balloon
out of the liquid nitrogen it has shrunk. The helium is
denser than the surrounding air. I set it on the table (dark
blue above) and it just sat there.
As the balloon of helium warms and expands its density decreases
(light blue). For a brief moment it has the same density as
the surrounding air (green). It's neutrally buoyant at this
point. Then it warms back to near room temperature where it
is again finds itself less dense than the air and lifts off the
table (yellow).
Free convection
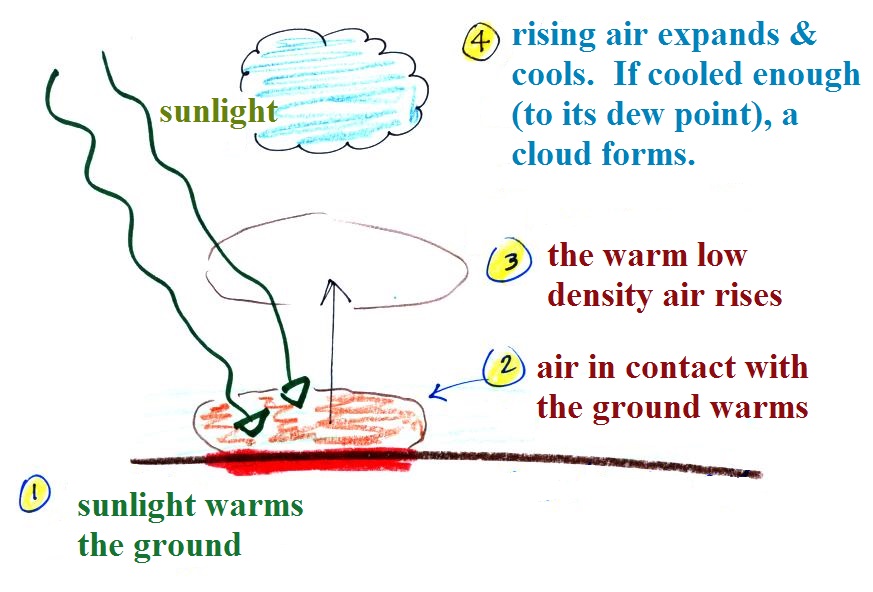
Something like this happens in the atmosphere.
Sunlight shines through the atmosphere. Once it reaches the
ground at (1) it is absorbed and warms the ground. This in
turns warms air in contact with the ground (2) As this air
warms, its density starts to decrease (pressure is staying
constant). When the density of the warm air is low enough,
small "blobs" of air separate from the air layer at the ground and
begin to rise, these are called "thermals." (3) Rising air
expands and cools (we've haven't covered this yet and it might
sound a little contradictory). If it cools enough (to the
dew point) a cloud will become visible as shown at Point 4.
This whole process is called convection; many of our summer
thunderstorms start this way.
Archimedes' principle
Here's another way of trying to understand why warm air
rises and cold air sinks - Archimedes Law or Principle (see pps
54a & 54b in the ClassNotes). It's perhaps a
simpler way of understanding the topic. A gallon bottle of
water can help you to visualize the law.
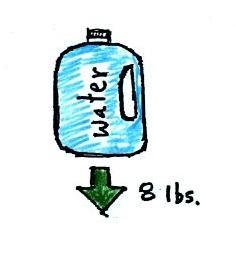
A gallon of water weighs about 8
pounds (lbs). I wouldn't want to carry that much water on
a hike unless I thought I would really need it.
Here's something that is kind of surprising.
If you submerge the gallon of water in a swimming pool, the
jug becomes, for all intents and purposes, weightless. The
weight of the water (the downward gravity force) doesn't just go
away. Once the jug is immersed, an upward force appears
and it is strong enough to cancel out gravity. Archimedes'
recognized that this would happen and was able to determine how
strong the upward force would be.
The strength of the upward buoyant force is the weight of
the fluid displaced by the bottle. In this case the 1
gallon bottle will displace 1 gallon of pool water.
One gallon of pool water weighs 8 pounds. The upward
buoyant force will be 8 pounds, the same as the downward
force. The two forces are equal and opposite.
What Archimedes law doesn't really tell you is what
causes the upward buoyant force. You should know what
the force is - it's the upward pressure difference force.
|
|
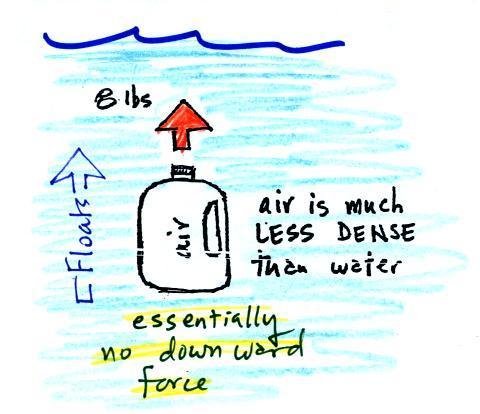
| We've poured out the water and filled
the 1 gallon jug with air. Air is much less dense
than water; compared to water, the jug will weigh
practically nothing. But it still displaces a gallon
of water and experiences the 8 lb. upward buoyant
force. The bottle of air would rise (actually it
shoots up to the top of the pool). |
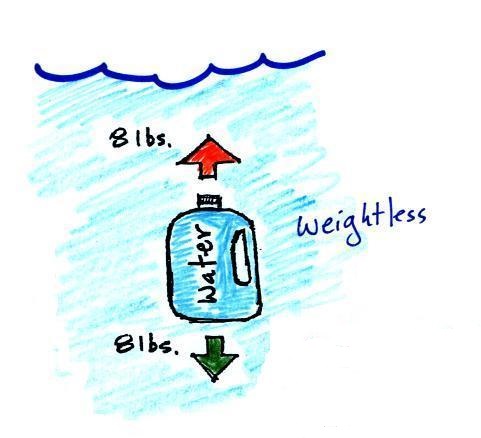
The density of the material inside and
outside the bottle are the same. A bottle
filled with water is weightless. |
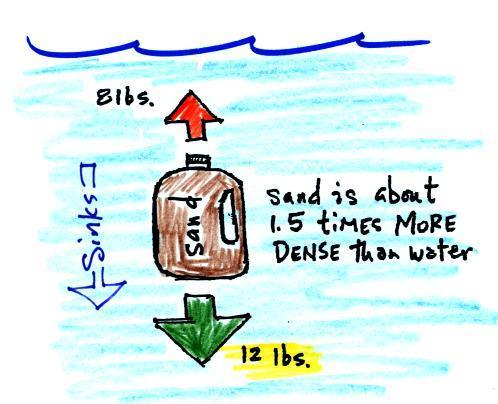
Next we'll fill the bottle with something
denser than water (I wish I had a gallon of mercury)
|
 |
| Sand is about 50% denser than
water. The weight of a gallon of sand is more
than a gallon of water. The downward force is
greater than the upward force and the bottle of sand
sinks. |
|
You can sum all of this up by saying anything
that is less dense than water will float in water, anything
that is more dense than water will sink in water.
Most types of wood will float (ebony and
ironwood will sink). Most rocks sink (pumice is an
exception).
The fluid an
object is immersed in doesn't have to be water, or a
liquid for that matter. You could immerse an
object in air. So we can apply Archimedes Law to
parcels of atmospheric air.

Air that is less dense (warmer) than the air around it will
rise. Air that is more dense (colder) than the air around
it will sink.
Here's a little more
information about Archimedes.
I want to show one last application of
some of what we have been learning - a Galileo
thermometer. It's a new acquisition of mine and fairly
fragile.
The left figure above comes from an
interesting and informative article in Wikipedia. The
right figure is a closeup view of the thermometer I brought to
class.
Here's an explanation of how the thermometers work. It
requires some time to process so I don't cover it in class
(details like this are not something you need to worry about but I
included them just in case you're curious).
The fluid in the thermometer will expand slightly if it
warms. It will shrink when it cools.
The changes in the volume of the fluid will change the fluid's
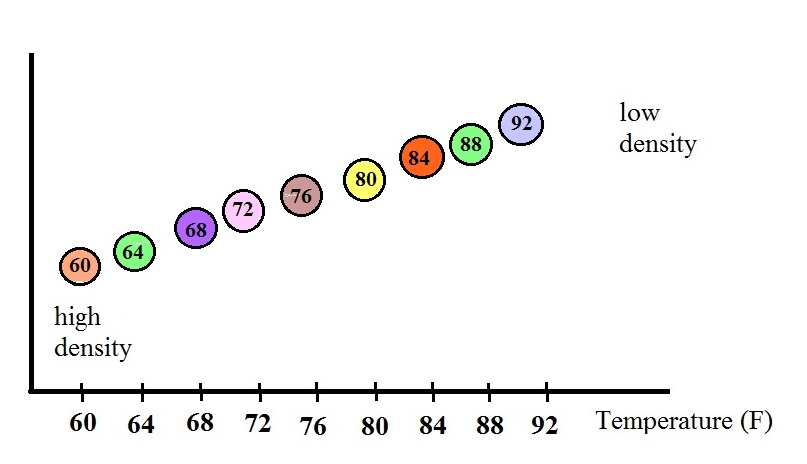
density. The graph above shows how the fluid density might
change depending on temperature. Note lower densities are
found near the top of the graph (the fluid expands as it warms).
The colored balls in the thermometer all
have slightly different densities. They also have little
temperature tags. The 60 F ball has a density equal to the
density of the fluid at 60 F. The 64 F ball has a
slightly lower density, the density of the fluid when it has
warmed to 64 , and so on. The densities of the floats don't
change.
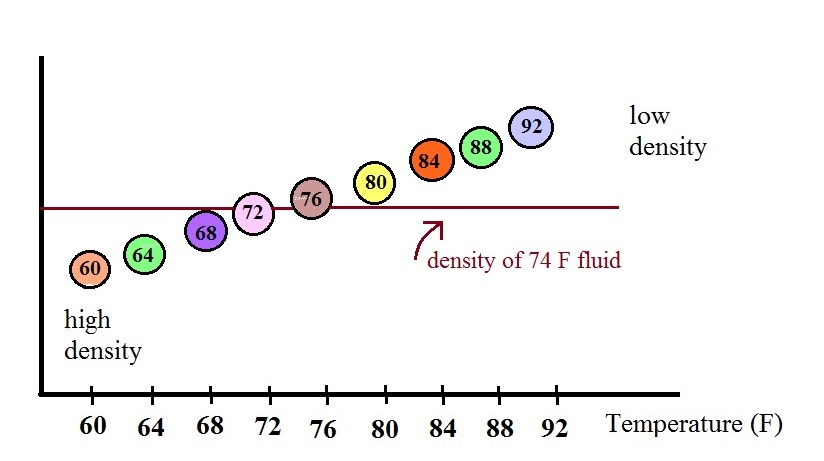
In use the density of the fluid in the thermometer will
change depending on the temperature. The densities of the
balls remain constant. As an example we will that the fluid
in the thermometer has a temperature of 74 F. The 60, 64,
68, and 72 F balls will all have densities higher than the fluid
(they lie below the 74F line in the graph above) and will
sink. The remaining balls have densities lower than the
fluid and will float.
The lower most floating ball in the illustration has a 76 F
temperature tag. The uppermost of the balls that have sunk
reads 72 F. The temperature is something between 72 F and 76
F. With this thermometer you can only determine temperature
to the nearest 4 F. Also the thermometer takes quite a while
(maybe an hour or two) to respond to a change in temperature.