
Good (stratospheric) and bad
(tropospheric) ozone
We'll first turn our attention to ozone.
Ozone has a kind of Dr.
Jekyll
and
Mr Hyde personality.

The figure above can be found on p.
14a in the photocopied ClassNotes. The ozone layer
(ozone in the stratosphere) is beneficial, it absorbs
dangerous high energy ultraviolet light (which would
otherwise reach the ground and cause skin cancer,
cataracts, and actually there are some forms of UV light
that would quite simply kill us).
Ozone in the troposphere is bad, it is toxic and a pollutant. Tropospheric ozone is also a key component of photochemical smog (also known as Los Angeles-type smog)
We'll be making some photochemical smog in a class demonstration. To do this we'll first need some ozone; we'll make use of the simple stratospheric recipe (shown above) for making what we need instead of the more complex tropospheric process (the 4-step process in the figure below). You'll find more details a little further down in the notes.
Ozone in the troposphere is bad, it is toxic and a pollutant. Tropospheric ozone is also a key component of photochemical smog (also known as Los Angeles-type smog)
We'll be making some photochemical smog in a class demonstration. To do this we'll first need some ozone; we'll make use of the simple stratospheric recipe (shown above) for making what we need instead of the more complex tropospheric process (the 4-step process in the figure below). You'll find more details a little further down in the notes.
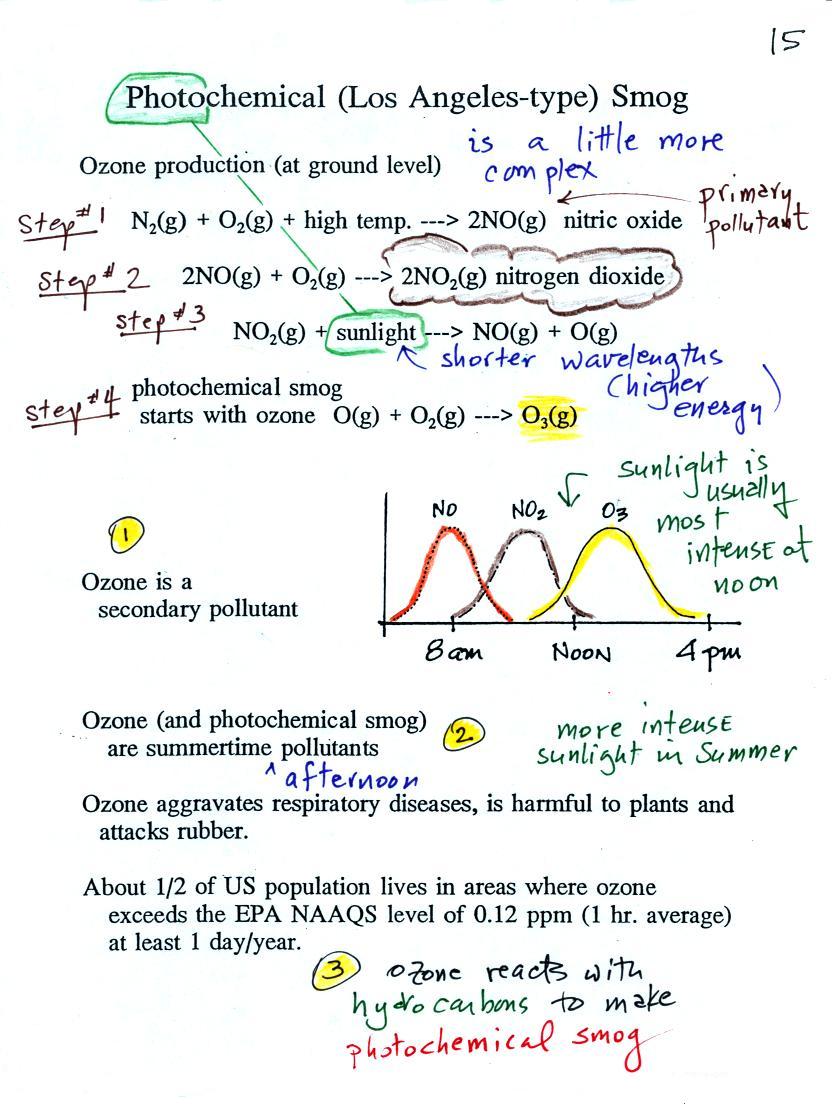
At the top of this figure
(p. 15 in the packet of ClassNotes) you see that a more
complex series of reactions is responsible for the
production of tropospheric ozone. The
production of tropospheric ozone begins with nitric oxide
(NO). NO is produced when nitrogen and oxygen in air
are heated (in an automobile engine for example) and
react.
The NO can then react with oxygen in the air to make nitrogen dioxide, the poisonous brown-colored gas that I used to make in class.
Sunlight can dissociate (split) the nitrogen dioxide molecule producing atomic oxygen (O) and NO. O and O2 react in a 4th step to make ozone (O3) just like happens in the stratosphere. Because ozone does not come directly from an automobile tailpipe or factory chimney, but only shows up after a series of reactions in the air, it is a secondary pollutant. Nitric oxide (NO) would be the primary pollutant in this example.
NO is produced early in the day (during the morning rush hour). The concentration of NO2 peaks somewhat later. Because sunlight is needed in step #3 and because sunlight is usually most intense at noon, the highest ozone concentrations are usually found in the afternoon. Ozone concentrations are also usually higher in the summer when the sunlight is more intense than at other times of year.
The NO can then react with oxygen in the air to make nitrogen dioxide, the poisonous brown-colored gas that I used to make in class.
Sunlight can dissociate (split) the nitrogen dioxide molecule producing atomic oxygen (O) and NO. O and O2 react in a 4th step to make ozone (O3) just like happens in the stratosphere. Because ozone does not come directly from an automobile tailpipe or factory chimney, but only shows up after a series of reactions in the air, it is a secondary pollutant. Nitric oxide (NO) would be the primary pollutant in this example.
NO is produced early in the day (during the morning rush hour). The concentration of NO2 peaks somewhat later. Because sunlight is needed in step #3 and because sunlight is usually most intense at noon, the highest ozone concentrations are usually found in the afternoon. Ozone concentrations are also usually higher in the summer when the sunlight is more intense than at other times of year.

Once ozone is formed, the ozone can
react with a hydrocarbon of some kind to make a product
gas. The ozone, hydrocarbon, and product gas are all
invisible, but the product gas sometimes condenses to make
a visible smog cloud or haze. The cloud is composed
of very small droplets or solid particles. They're
too small to be seen but they are able to scatter light -
that's why you can see the cloud.
Here's a pictorial summary of the photochemical smog demonstration.
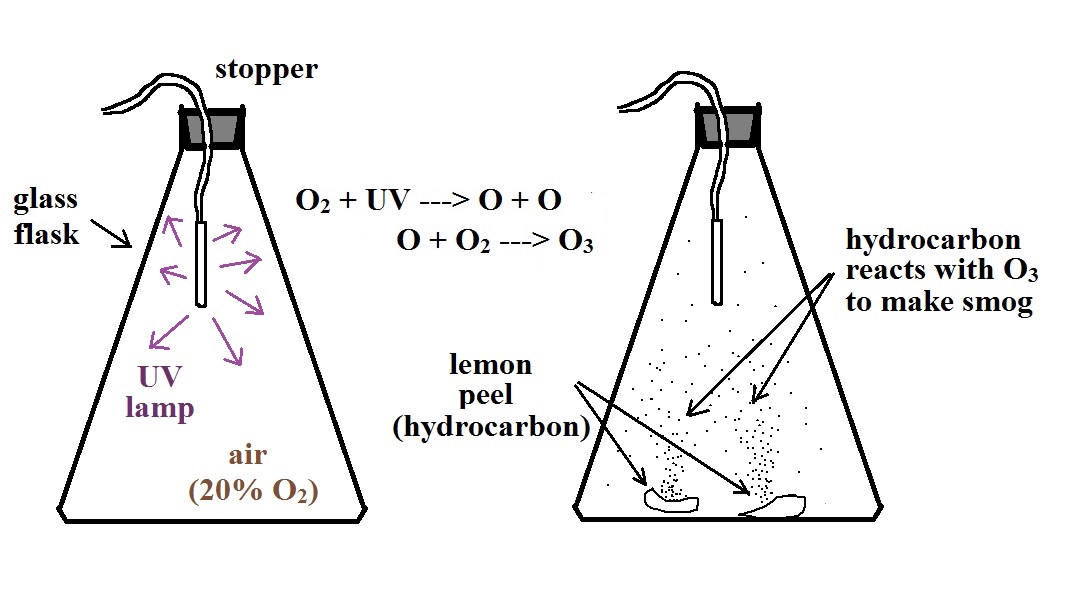
We started by putting a small "mercury vapor" lamp inside a flash. The bulb produces a lot of ultraviolet light (the bulb produced a dim bluish light that we could see, but the UV light is invisible so we had no way of really telling how bright the bulb was). The UV light and oxygen in the air produced a lot of ozone (you could have easily smelled it if you had taken the cover off the flask).
After a few minutes we turned off the
lamp and put a few pieces of lemon peel into the
flash. Part of the smell of lemon is limonene, a
hydrocarbon. The limonene gas reacted with the ozone
to produce a product gas of some kind. The product
gas condensed, producing a visible smog cloud We
shined the laser beam through the smog cloud to reinforce
the idea that we are seeing the cloud because the drops or
particles scatter light.
O2 + spark ---> O + O
One of the oxygen atoms reacts with an oxygen molecule to form O3
O + O2
---> O3
The smog cloud produced in the video is a little thicker than the one produced in class. I suspect that is because they first filled the flask with pure oxygen, 100% oxygen, before making the ozone. I used air in the room which is 20% oxygen. More oxygen in the flask means more ozone and a thicker cloud of Los Angeles type smog.
Back to our summary list

Sulfur dioxide and acid rain
Sulfur
dioxide is one of the pollutants that can
react with water in clouds to form acid rain
(some of the oxides of nitrogen can also
react with water to form nitric acid).
The formation and effects of acid rain are
discussed on p. 12 in the photocopied Class
Notes.
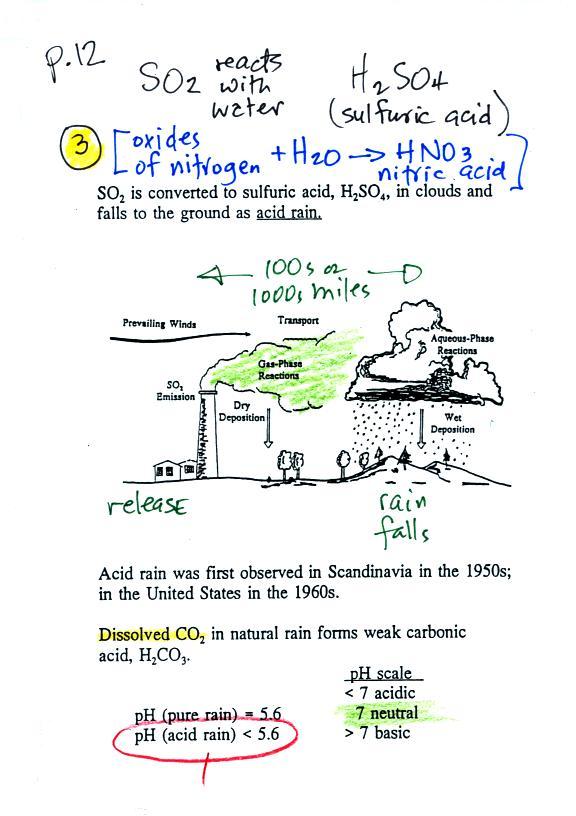
Acid rain is often a problem in
regions that are 100s even 1000s of miles from the
source of the sulfur dioxide. Acid rain in Canada
could come from sources in the US, acid rain in
Scandinavia came from industrialized areas in other
parts of Europe.
Note at the bottom of the figure above that natural "pristine" rain has a pH less than 7 and is slightly acidic. This is because the rain contains dissolved carbon dioxide gas. The acid rain demonstration described below and done in class should make this point clearer.
Note at the bottom of the figure above that natural "pristine" rain has a pH less than 7 and is slightly acidic. This is because the rain contains dissolved carbon dioxide gas. The acid rain demonstration described below and done in class should make this point clearer.
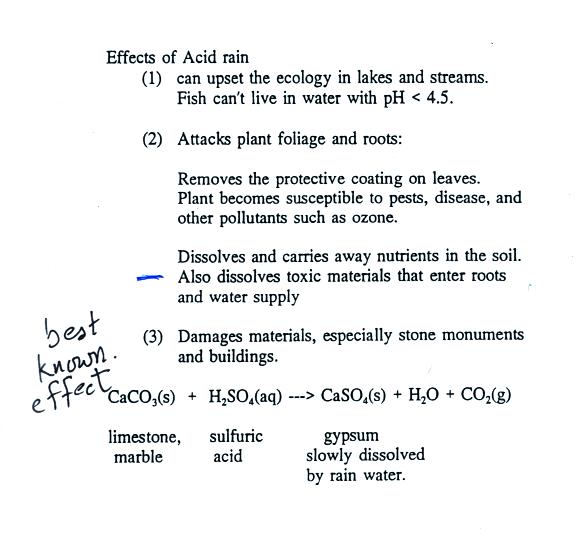
Some of the problems associated with acid rain are listed above. We'll start class on Wednesday with a short "acid rain" demonstration. In the meantime here's a summary of the main points to remember concerning sulfur dioxide.






