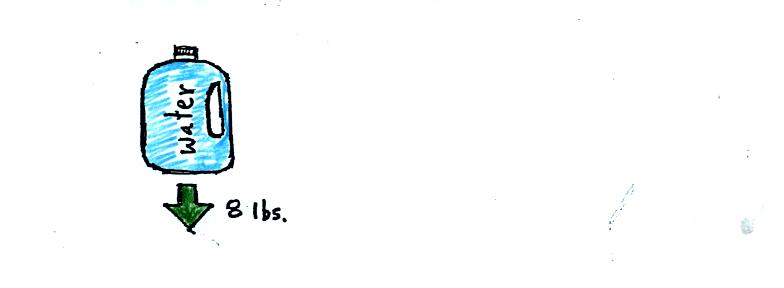Friday Feb. 29!, 2008
The 1S1P Topic #3 reports were returned in class today. All of
the 1S1P Assignment #1 reports have now been graded. Copies of
the 1S1P Assignment #2 worksheets were distributed in class.
An in-class Optional Assignment was also distributed in class.
The assignment was turned in at the end of class.
Here's a
little story to start out NATS 101 today.
Chapter 1
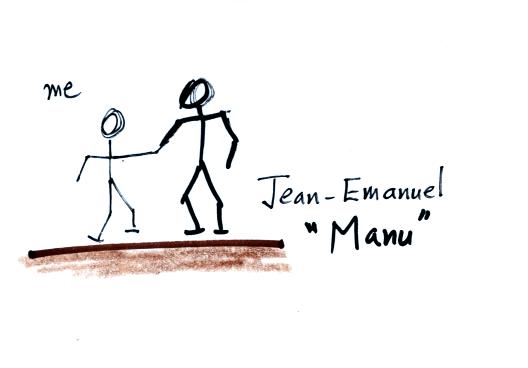
About 20 years ago the instructor of this class lived and
worked in France for a short time (you might remember hearing about his
Peugeot 404 auttomobile earlier in the semester). One of the
people he met there
was a big solidly constructed guy named Jean-Emanual (perhaps
Jean-Emmanuel). If you met Manu,
as he was more commonly called, at a party and if he had had a little
bit to drink he would sometimes greet you by shaking your hand
and saying "I could crush you" (he'd say it in English to me just to be
sure I understood him). Now so far as I know Manu never really
did crush anyone, but something like that makes quite an impression.
Why on earth is Mr. Weidman telling this story, has he been
drinking? Did he suddenly get the idea that
he could crush us (the students in NATS 101)?
Click here to read Chapter 2.
The next topic was Archimedes
Law of Bouyancy. You'll find this discussed on pps 53a and 53b in the
photocopied Classnotes.
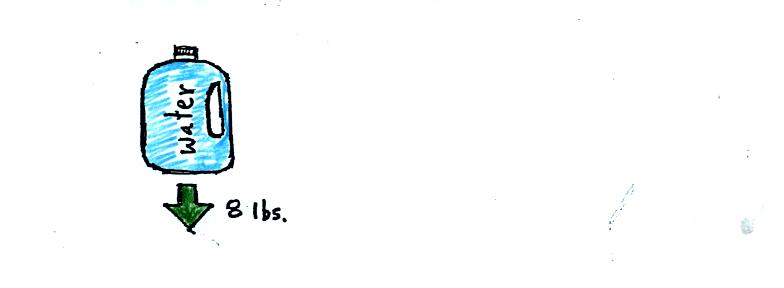
A gallon of water weighs about 8 pounds (lbs). Density is mass
divided by volume; if someone were to ask you what the density of water
was you could respond "8 pounds per gallon." Strictly speaking
pounds aren't units of mass, but we aren't going to worry about that
technicality here.
If you submerge a 1 gallon jug of water in a swimming pool, the jug
becomes, for all intents and purposes, weightless. Archimedes'
Law (see figure below) explains why this is true.

The jug will displace 1 gallon of pool water. One gallon of pool
water weighs 8 pounds. The upward bouyant force will be 8 pounds,
the same as the downward force on the jug due to gravity. The two
forces are equal and opposite.
Now we imagine pouring out all the water and filling the 1 gallon jug
with air. Air is about 1000 times less dense than water; the jug
will weigh practically nothing.
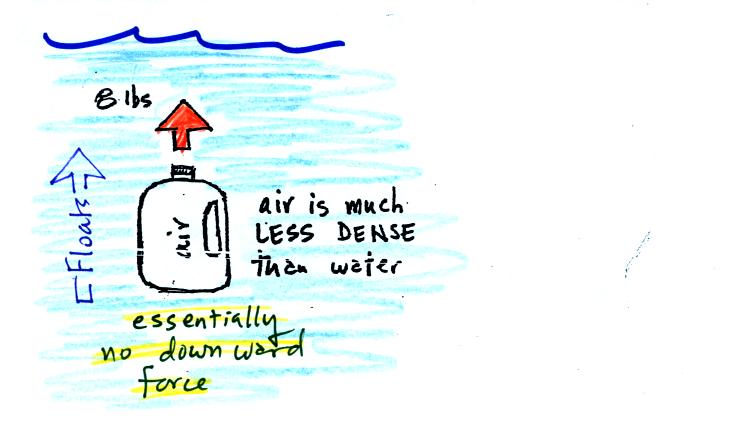
If you submerge the jug in a pool it will displace 1 gallon of water
and experience an 8 pound upward bouyant force again. Since there
is no downward force the jug will float.
One gallon of sand (which is about 1.5 times denser than water) jug
will weigh 12 pounds.
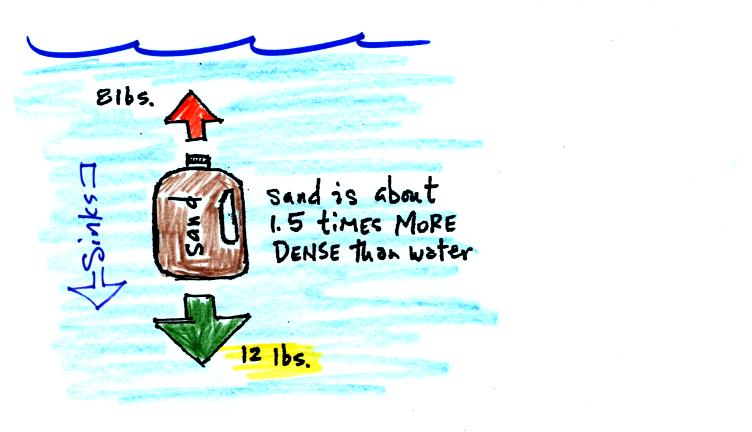
The jug of sand will sink because the downward force is greater than
the upward force.
You can sum all of this up by saying anything that is less dense than
water will float in water, anything that is more dense than water will
float in water.
The same reasoning applies to air in the atmosphere.
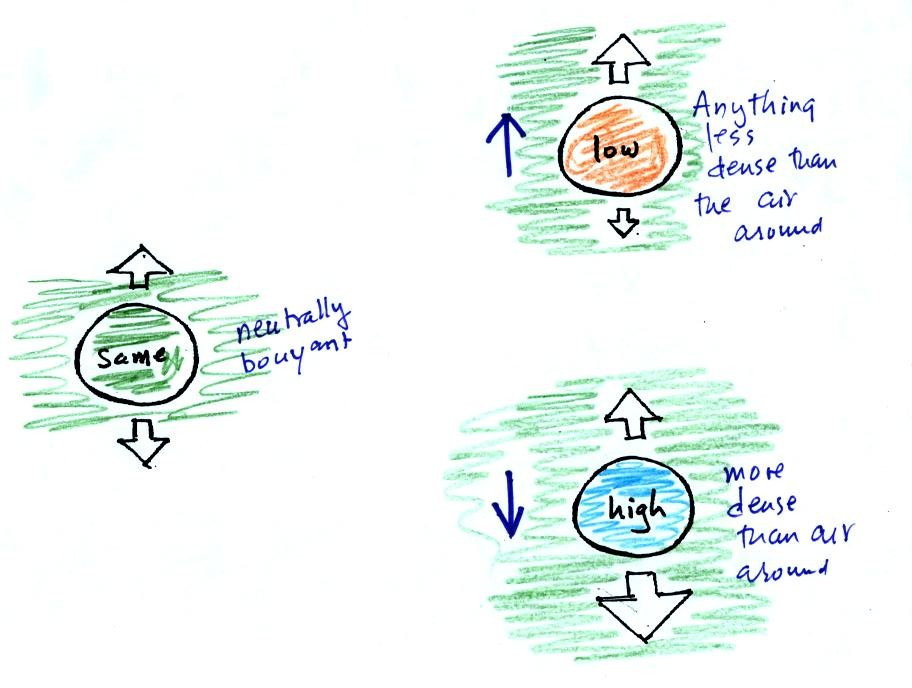
Air that is less dense (warmer) than the air around it will rise.
Air that is more dense (colder) than the air around it will sink.
Here's a little more
information about Archimedes which
wasn't covered in class.
It was time for a colorful demonstration involving water and objects
that either float or sink in water.
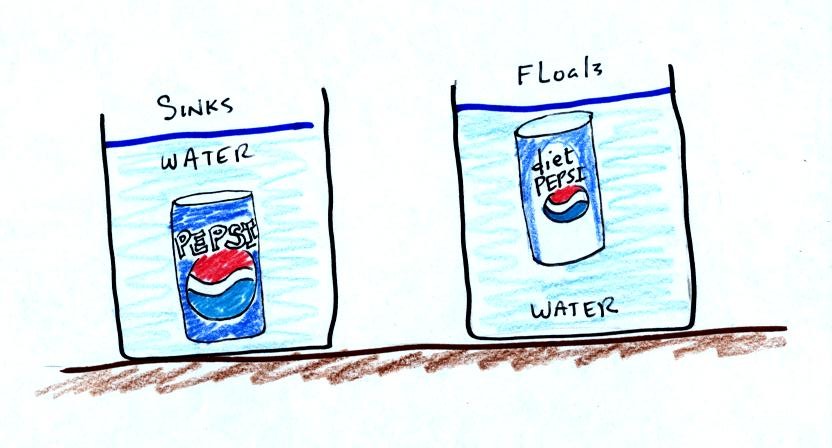
A can of regular Pepsi was placed in a beaker of water. The can
sank.
Both cans are made of aluminum which has a density almost three times
higher than water. The drink itself is largely water. The
regular Pepsi also has a lot of sugar or corn syrup, the diet Pepsi
doesn't. The mixture has a density greater than plain
water. Both cans contain a little air (or perhaps carbon dioxide
gas). This is much less dense than water.
The average density of the can of regular Pepsi (water&sugar +
aluminum + air) ends up being slightly greater than the density of
water. The average density of the can of diet Pepsi (water +
aluminum + air) is slightly less than the density of water.
In some respects people in swimming pools are like cans of regular and
diet Pepsi. Some people float (they're a little less dense than
water), other people sink (slightly more dense than water). The next two figures weren't shown in
class.

Many people can fill their lungs with air and make themselves float, or
they can empty their lungs and make themselves sink.
People must have a density that is about the same as water.

The course instructor has gained some weight over the winter and weighs
160 pounds. If you assume he has a density about equal to water,
8 pounds per gallon, you find that the course instructor has a volume
of about 20 gallons.
Latent
heat energy transport was the final topic of the day.
Energy
transport in the form of latent heat is the second most important
energy transport process (second only to electromagnetic
radiation). This process is sometimes a little hard to visualize
or understand because the energy is "hidden" in water vapor or water.
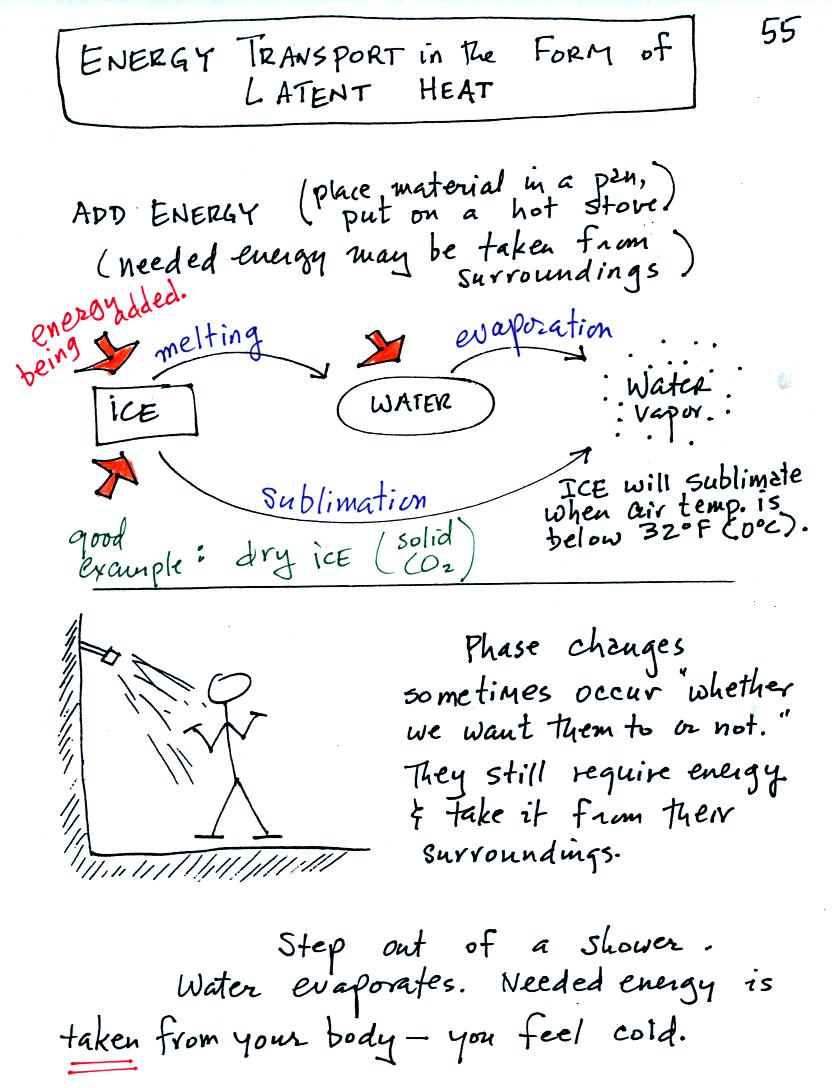
Latent heat energy transport is associated with changes of
phase (solid to liquid, water to water vapor, that sort of thing) A
solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously add or supply the energy (such as when you put
water in a
pan and put the pan on a hot stove) or the needed energy will be
taken from the surroundings (from your body when you step out of a
shower in the morning).
Some dry ice was displayed on the projector screen.
Dry ice is solid carbon
dioxide and is very cold. Dry ice sublimates, it changes directly
from solid carbon dioxide to carbon dioxide gas. Energy must be
added to the dry ice in order for it to sublimate. You were asked
(on the in-class Optional Assignment) to look closely at the dry ice
and identify two energy transport processes that you could actually see
in operation.
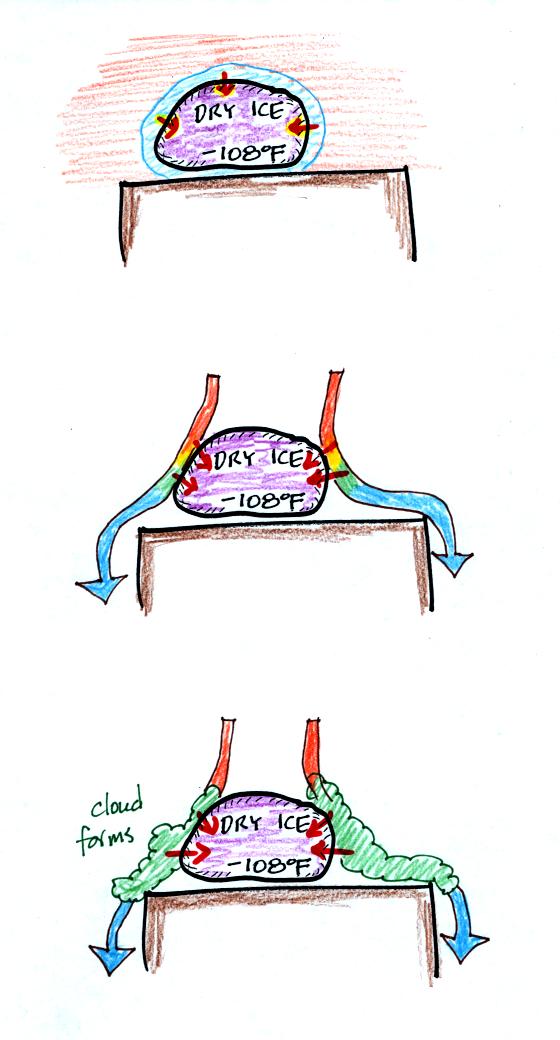
In the top figure, conduction transports energy from a thin layer of
warm air in contact with the dry ice to the dry ice. You can't
really see this process.
In the middle figure warm air coming into contact with the dry ice
looses energy and cools. This cool air sinks and is replaced by
warmer air. This process, free convection, is transporting energy
to the dry ice. Because you could actually see these sinking air
motions, this is one of the answers to the question.
Finally in the bottom figure, air that comes into contact with the dry
ice is cooled to the dew point and a cloud forms. You could
actually see the cloud. The cloud is produced by moisture in the
air, it isn't carbon dioxide gas. As we will see the formation of
the cloud, a phase change, is an additional source of energy.
This would be an example of latent heat energy transport. This is
the second energy transport process you should have identified.
A bottle
containing solid iodine crystals was also passed around class together
with a bottle filled only with air. The crystals of iodine were
sublimating, changing from solid to gas. What
is unusual however is that the iodine gas is visible. The bottle
containing the iodine has a just barely visible pink or purple
color. You can see a
picture of iodine and iodine gas at the webelements.com website.

A 240 pound man (or woman) running at 20 MPH has just enough
kinetic energy (if you could somehow capture it) to
be able to melt an ordinary ice cube. It would take 8 people to
evaporate the resulting water.
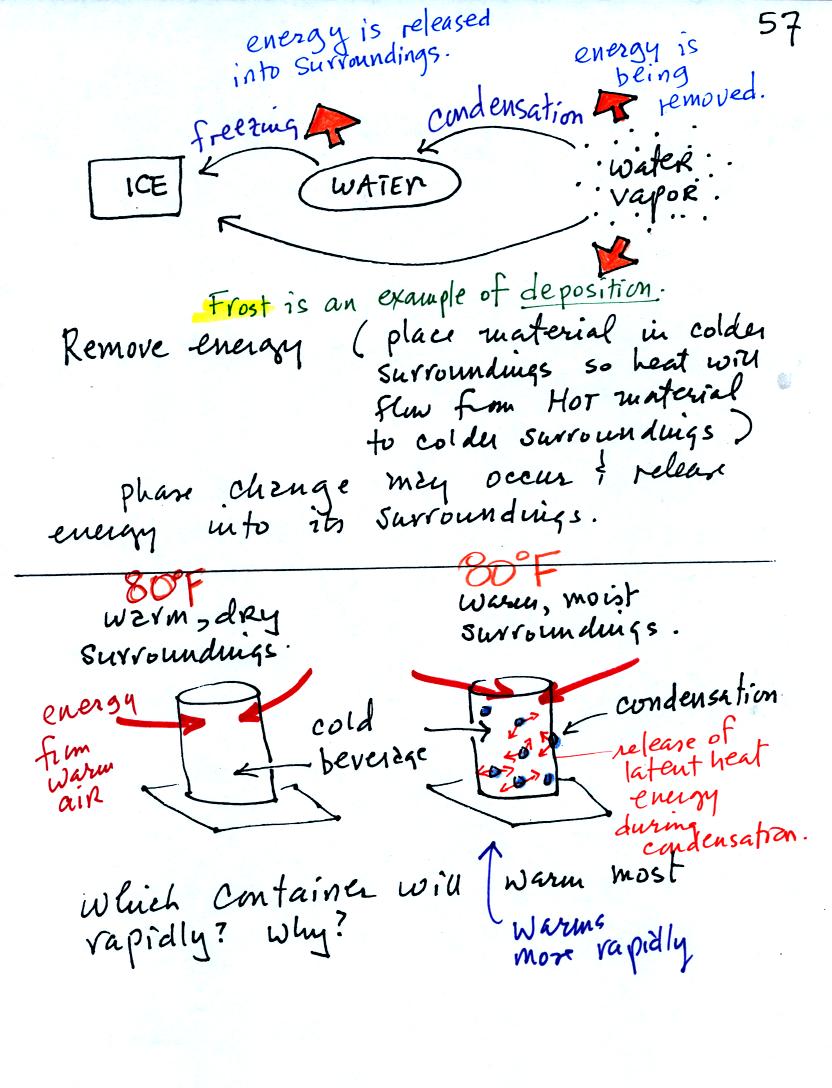
You can consciously remove energy from water vapor to make
it
condense
or from water to cause it to free (you could put water in a
freezer; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm). Note the orange energy arrows have
turned around and are pointing from the material toward the
surroundings.
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more
rapidly. The condensation may actually be the dominant process.

The story starts at left in the
tropics where there is often an abundance or surplus of energy;
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.