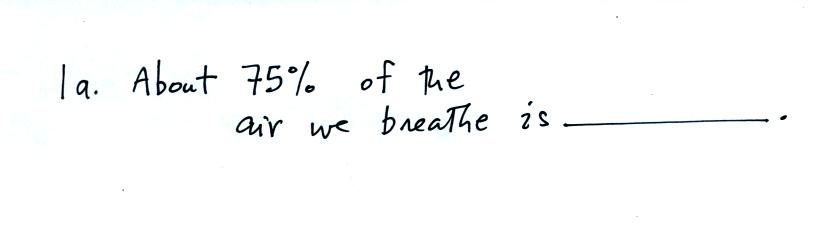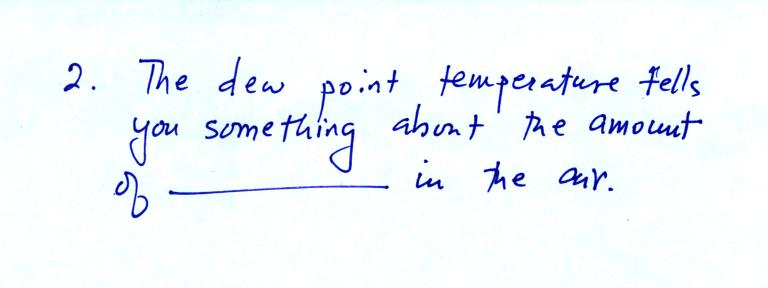Wednesday
Jan. 16, 2008
The
first day of class.
We first briefly discussed the Course
Information
handout. Read through this carefully on your own. Note the
various options you have for purchasing a copy
of the course textbook. You should try to purchase a copy of the
photocopied
Classnotes (in the bookstore) right away as we will probably be using
some of
them in class on Friday.
Next we looked at the Writing
Requirements
handout. You should be thinking about which of the 4 experiments
(or book or scientific paper reports) you would like to do so that you
can sign up in class on Friday. Distribution of the materials
for
the first experiment will probably also begin in class on Friday.
Your grade
in this class will depend on your quiz scores, how much
extra credit you earn, your writing grade, and (perhaps) your
score on the final exam. A sample grade report from the Spring
2007 MWF Nats 101 class was shown.
Doe_J
quiz1 -36 (160 pts possible)
77.5%
quiz2 -46 (155 pts possible)
70.3%
quiz3 -48 (165 pts possible)
73.1%
quiz4 -35 (160 pts possible)
78.1%
2.6 EC points (3.3 pts possible)
writing scores: 33.0 (expt/book report) + 45.0
(1S1P pts)
writing grade: 97.5%
average (no quiz scores dropped): 79.3% + 2.6 =
81.9%
average (lowest quiz score dropped): 81.6% + 2.6 =
84.2
you DO need to take the final exam
-24.8 pts missed on the final exam = 75.3%
3Q&W>F overall average is 82.4
Don't
worry about all the details at this point. Note that this student
earned C grades on all the quizzes and the final exam but ended up with
a B in the class. This is due largely to the high writing grade
and the extra credit points.
We'll
begin this new semester in Chapter 1 of the text. Before
opening
the book and beginning the first reading
assignment, try to imagine
what you would put in the first chapter of a meteorology and
climatology textbook.

Student
answers to the question above included: temperature of the
outside air, whether there was enough oxygen in the outside air to
breathe. These and other basic characteristics of the atmosphere
such as air pressure and air density are covered in Chapter 1.
Today we
were mostly just concerned with the composition of the earth's
atmosphere, in
particular the 5 most abundant gases in the earth's atmosphere.
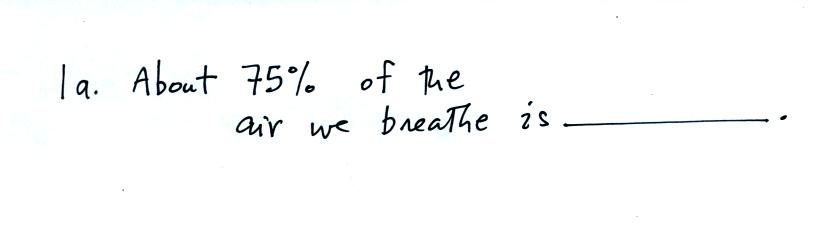
This
is the first of several questions asked during class. It was an
anonymous survey.
Some
of
this material, in liquid form, was poured into a styrofoam cup.

Class
survey results: 65% said nitrogen, 20% chose
carbon dioxide, and 11% said oxygen.
The correct answer is
nitrogen (you can fill in the blank in Question 1a and in the figure
above with the word
nitrogen). You can see liquid nitrogen, it is clear (not blue as
shown above) and looks
like
water. Once it has
evaporated and turned into a gas it is invisible. Nitrogen was
discovered in 1772 by Daniel Rutherford (a Scottish
botanist). Atmospheric nitrogen is relatively unreactive
and is sometimes used to replace air in packaged foods to preserve
freshness.
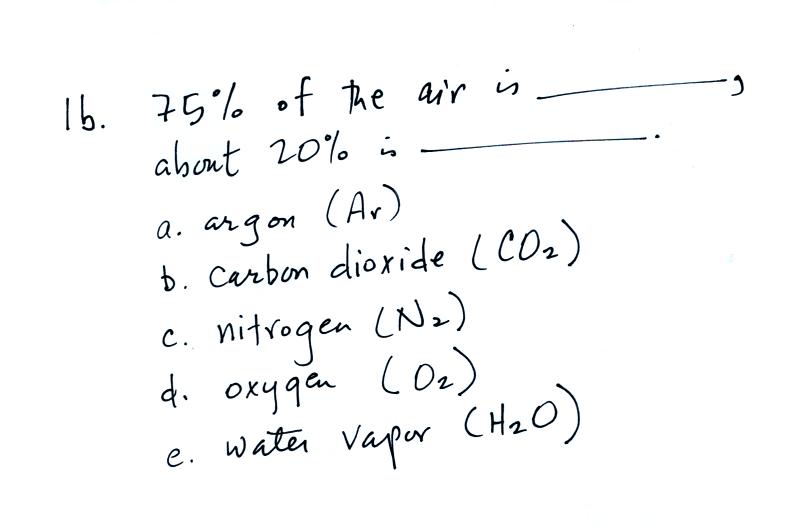
Here's
the same question plus an additional question. Multiple choice
questions like this might be easier to answer than a fill-in-the-blank
question.
Fill in
the first blank with nitrogen; oxygen goes in the 2nd blank. Oxygen
is
the second most abundant gas in the atmosphere. Oxygen is the
most abundant element (by mass) in the earth's crust, in ocean water,
and in the human body. Here's
a photograph of liquid oxygen. It really does have a blue
(light blue) color.
When heated (such as in an automobile engine) the oxygen and
nitrogen in air react
to form compounds such as nitric oxide (NO), nitrogen dioxide (NO2),
and nitrous oxide (N2O). Together as a group these are
called oxides of nitrogen; the first two are air
pollutants, the
last is a greenhouse gas.
The 5 most abundant gases in the atmosphere were listed.

Water vapor and argon are the 3rd and 4th most abundant gases in the
atmosphere. The concentration of water vapor can vary from near
0% to as high as 3% or 4%. Water is, in many locations, the 3rd
most abundant gas in air. In Tucson, the air is often dry enough
that argon is in 3rd position and water vapor is 4th.
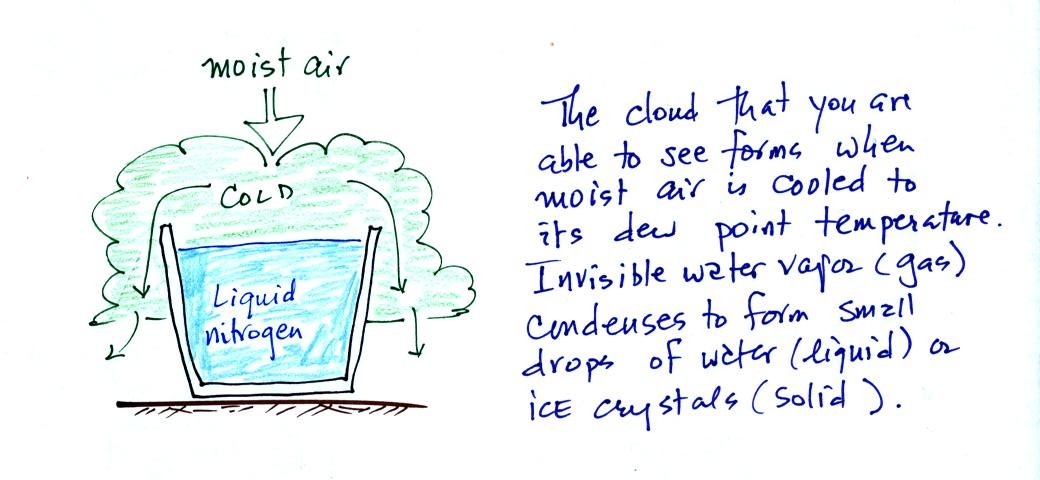
Water vapor, a gas, is
invisible. Clouds are visible because they are made up of small
drops of liquid
water or ice crystals. Water is the only compound that exists
naturally in solid, liquid, and gaseous phases in the atmosphere.
Argon is an unreactive noble gas (helium, neon, krypton, xenon, and radon are also inert gases).
Noble bases are often used in "neon signs."
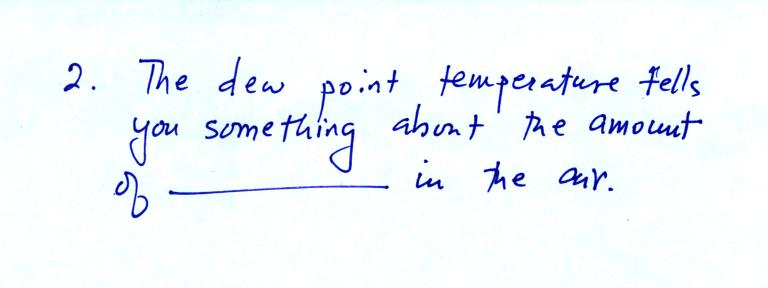
The answer to this question is water vapor.
Water plays an important role in the formation of clouds,
storms,
and weather. Meteorologists are very interested in knowing and
keeping track of how
much water vapor is in the air at a particular place and time.
One of the variables they use is the dew point temperature; it has two
"jobs."

Its first job is to provide
a measure of the amount of water
vapor in the air. The dew point is just a number. When
the value
is low the air doesn't contain much moisture. The higher the dew
point value, the more water vapor there is in the air.
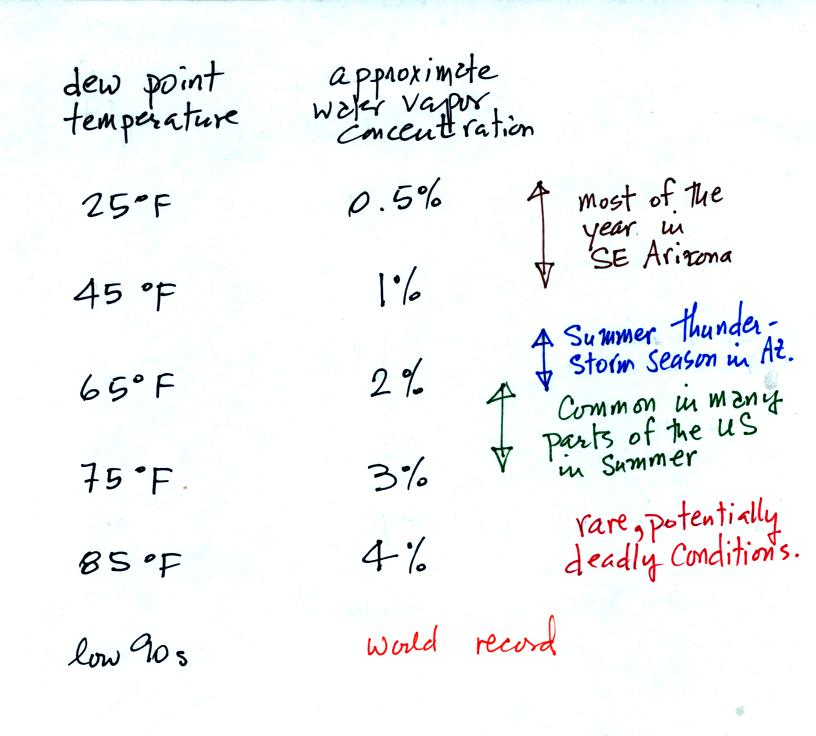
The chart below gives a rough equivalence between dew point
temperature and percentage concentration of water vapor in the air.
Click here
to see current dew point temperatures across the U.S.
Note added Thursday Jan. 17.
The dew point in Tucson this morning (before the 8 am section of class)
was 2o F. This is very low, the air is currently very
dry.
Even drier air can be found in the "4 Corners" area where dew points of
-10o F, even -20o F are being observed.
The second job of the dew point temperature is illustrated
below. When you cool moist air to its dew point, the
relative humidity becomes 100% and a cloud forms.
We will
cover each of the topics a-d below at the beginning of this
semester. This is an election year and Arizona's presidential
primary election is Feb. 5, part of "super Tuesday." In that
spirit the class will decide which topic to cover first. You'll
learn the results in class on Friday.

The following material wasn't covered
in class.
Our present
atmosphere is very different from the earth's
original atmosphere.
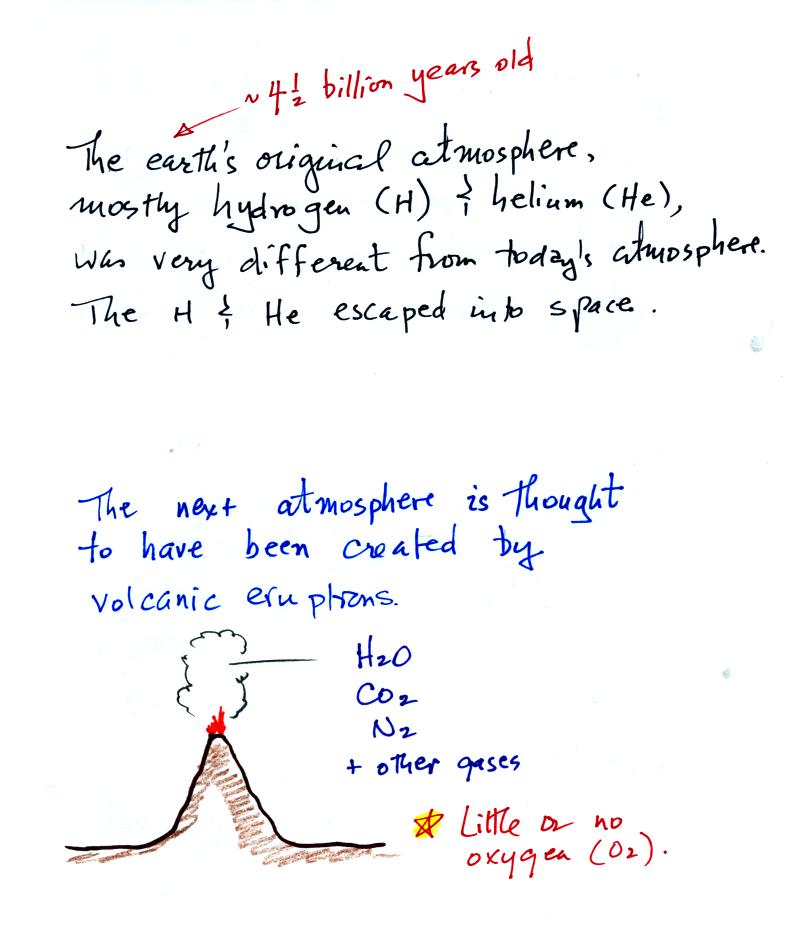
The earth's first atmosphere was composed mainly of
hydrogen
and
helium.
These light-weight gases escaped into space and were lost. The
next atmosphere was built up of gases emitted during volcanic
eruptions, mostly water vapor, carbon dioxide, and nitrogen. As
the earth began to cool the water vapor condensed and began to create
oceans. Carbon dioxide dissolved in the oceans and was slowly
turned into rock. Much of the nitrogen remained in the atmosphere.
Volcanoes didn't add any of the oxygen that is the atmosphere.
Where did that come from?
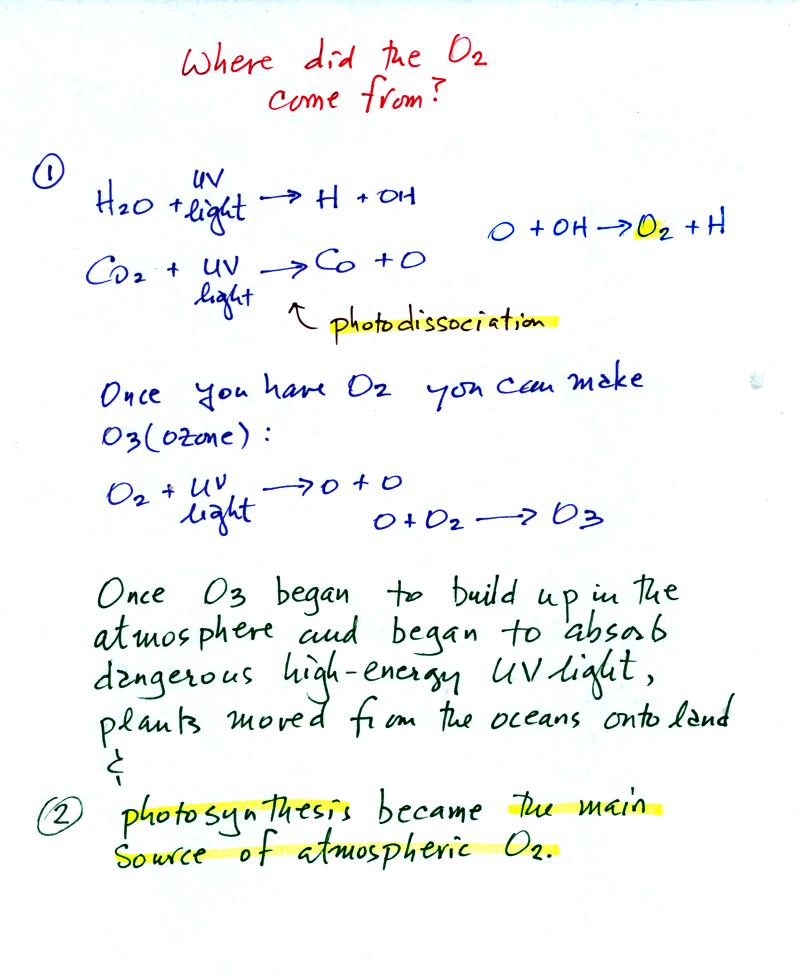
The oxygen is thought to have first come from
photodissociation of
water vapor and carbon dioxide by ultraviolet light (the high energy
radiation splits the H20 and CO2 molecules into
pieces). The O and OH react
to form O2 and H.
Once O2 begins to accumulate in the air it can react with O
to form
ozone, O3. The ozone then begins to absorb ultraviolet
light and life forms can safely move from the oceans (which would
absorb UV light in the
absence of ozone) onto land. Eventually plants and photosynthesis
would become the main source of atmospheric oxygen.