Wednesday Jan. 30, 2008
The Practice Quiz is one week from today. A Practice Quiz Study Guide is now available
online. Study guides should also appear about one week before
future quizzes this semester. Click here
is you would like to download the study guide in Microsoft WORD format
for printing. You will find sample questions listed on the Study
Guide taken from Fall 2000 NATS 101 quizzes. Click here to select and
download copies of those quizzes.
The following announcement was made at the beginning of class:
At least one student
in class is in need of a note-taker. If you feel you take
clear, concise notes and are willing to share a copy with a student
with a disability, please come to the front of the classroom at the end
of class so that you can meet the student. You will be provided
with arbonless copy paper to take notes and will be formally recognized
through a letter of volunteeer service for your portfolio or resume.
We are ready to move back
into the middle part of Chapter 1. We will be looking
at how atmospheric characteristics such
as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we first need to understand
what pressure is and what can cause it to change.
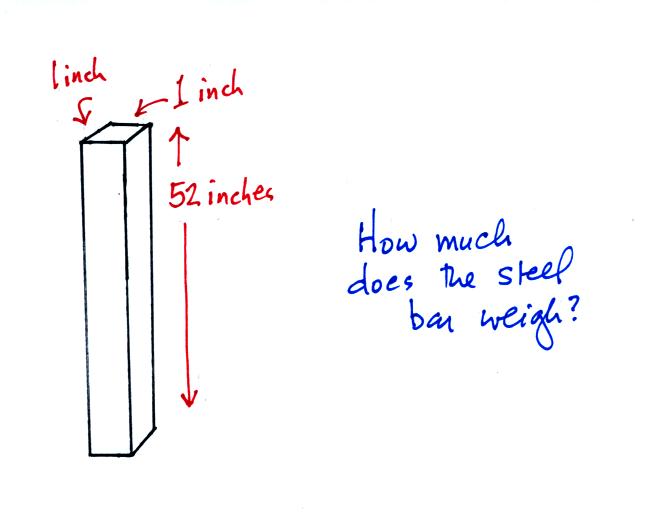
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We come back to the iron bar later in the class.
What
follows is a little more detailed
discussion of the basic concepts of mass, weight, and density
than is found on p. 23 in the photocopied Classnotes.
Before we can learn about atmospheric pressure, we
need to review
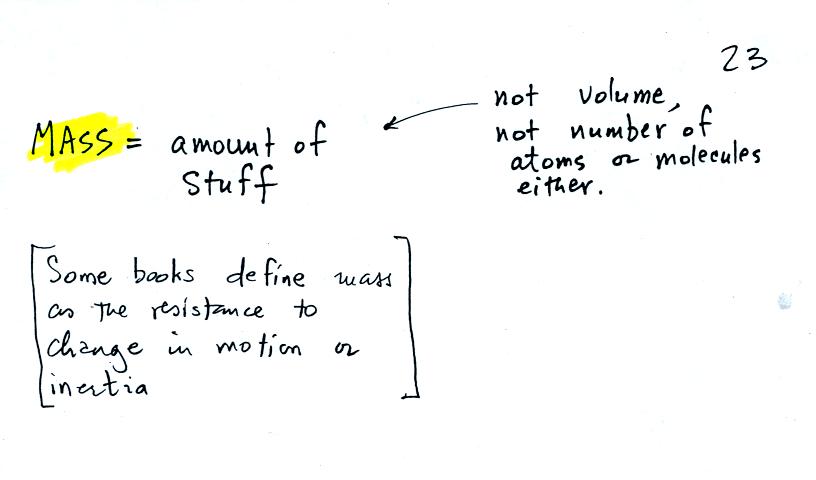
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder
to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down if it were
already moving).

It is possible to have two objects with the same
volume but very
different masses. The bottles of water and mercury that were
passed around class on Monday were an example (thanks for being careful
with the mercury).

To understand why there is such a
difference in mass and weight you need to look at the water molecules
and mercury atoms on an atomic scale.
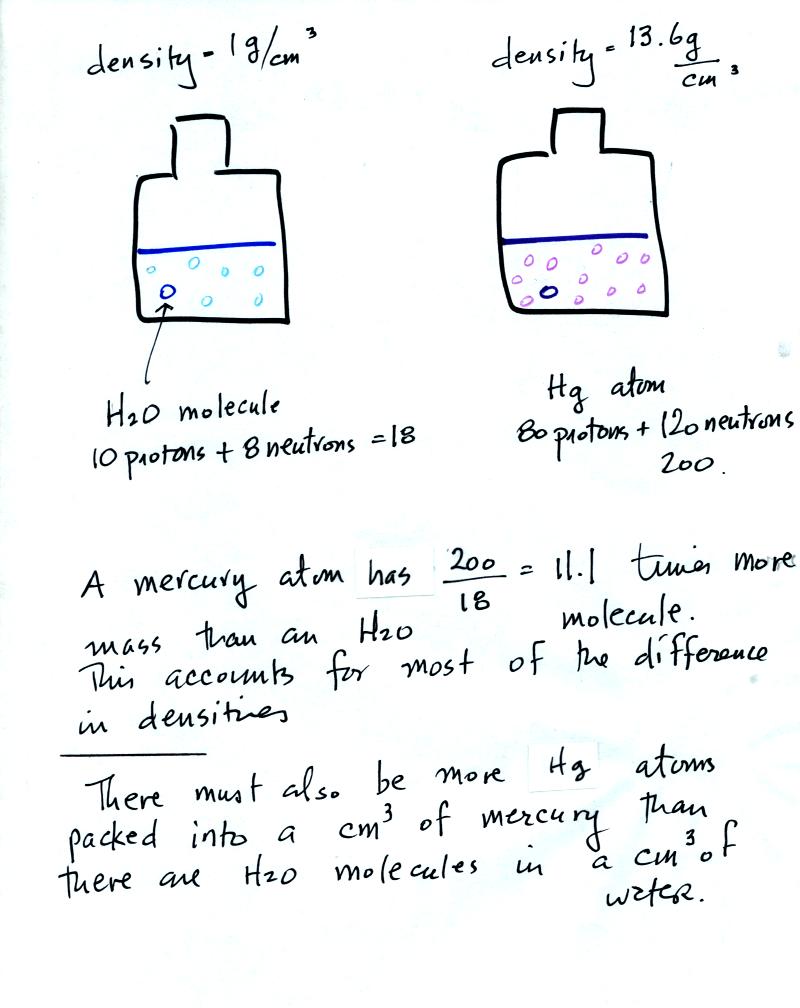
Mercury atoms are built up of many
more protons and neutrons
than a water molecule (also more electrons but they don't have nearly
as much mass as protons and neutrons). The mercury atoms have
11.1 times as much mass as the water molecule. This doesn't quite
account for the 13.6 difference in density. Despite the fact that
they contain more protons and neutrons, the mercury atoms must also be
packed closer together than the molecules in water.
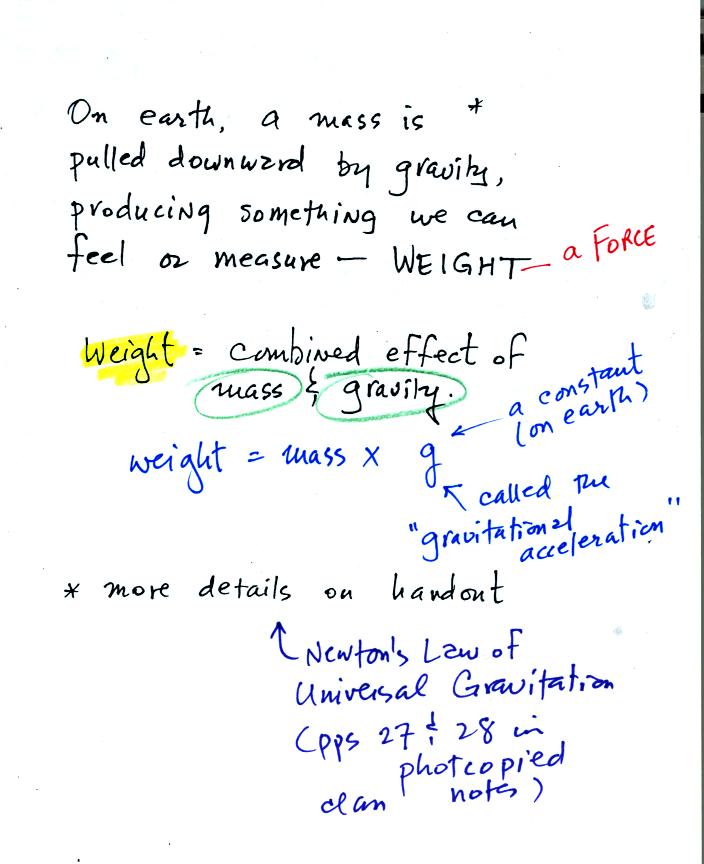
Weight
is a force and depends on
both the mass of an object and the
strength of gravity.
We tend to use weight and mass
interchangeably
because we spend all our
lives on earth where gravity never changes.

Any three objects that all have the same mass
would
necessarily have the same weight. Conversely
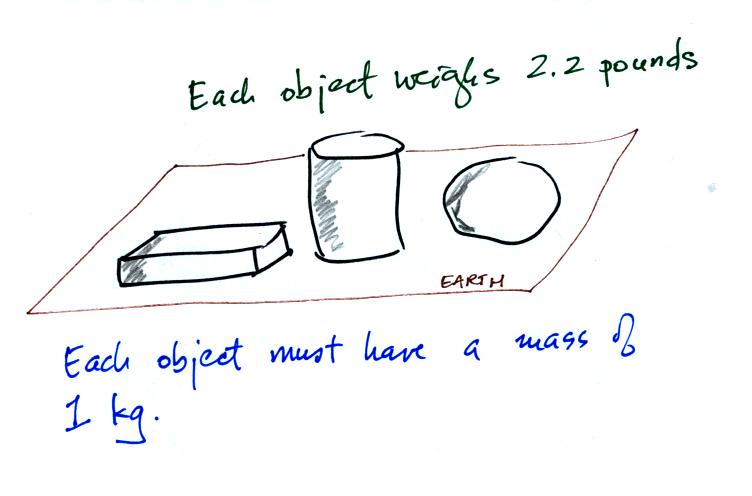
Three objects with the
same weight
would also have the same mass.
The difference between mass and weight is clearer
(perhaps) if you
compare the situation on the earth and on the moon.

If you carry an object
from the
earth to the moon, the mass
remains the
same (its the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.

Definition and
illustrations
of
high and low density.
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment to prove that air has weight.
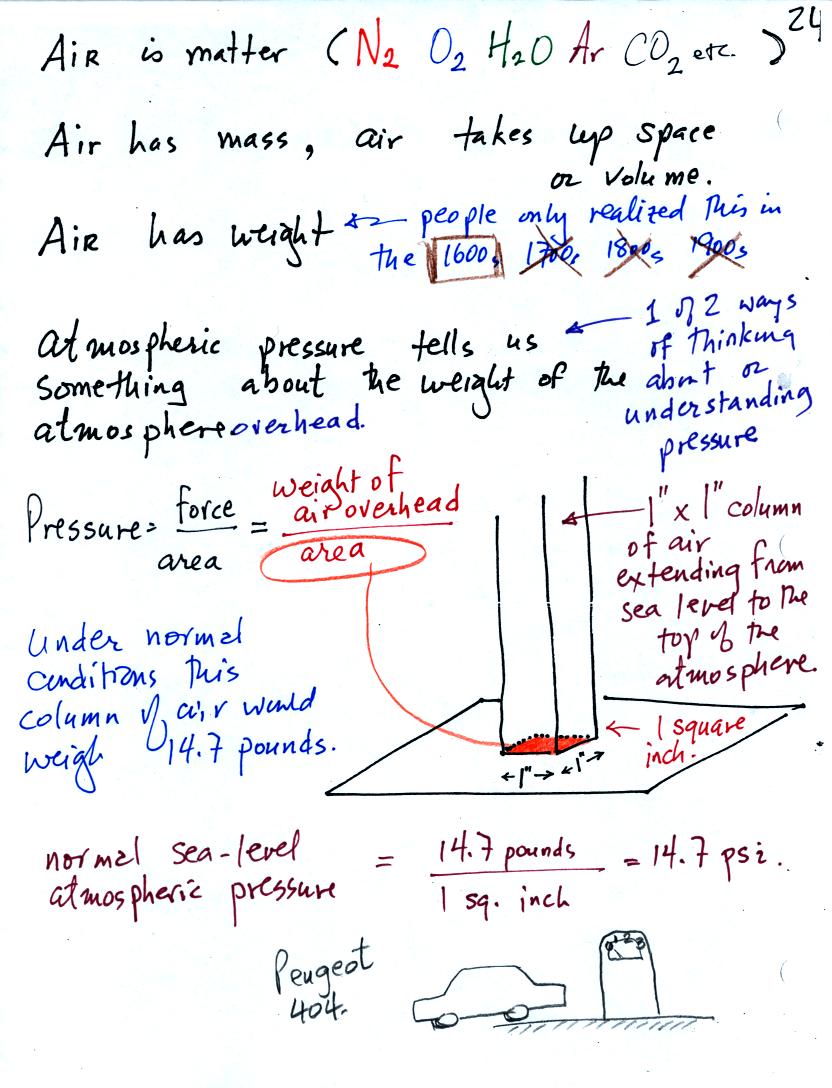
Pressure is defined as force divided by area. Air pressure is the
weight
of the atmosphere overhead divided by area the air is resting on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead.
Under normal conditions a 1 inch by 1 inch column of air stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your car or bike tires with air).
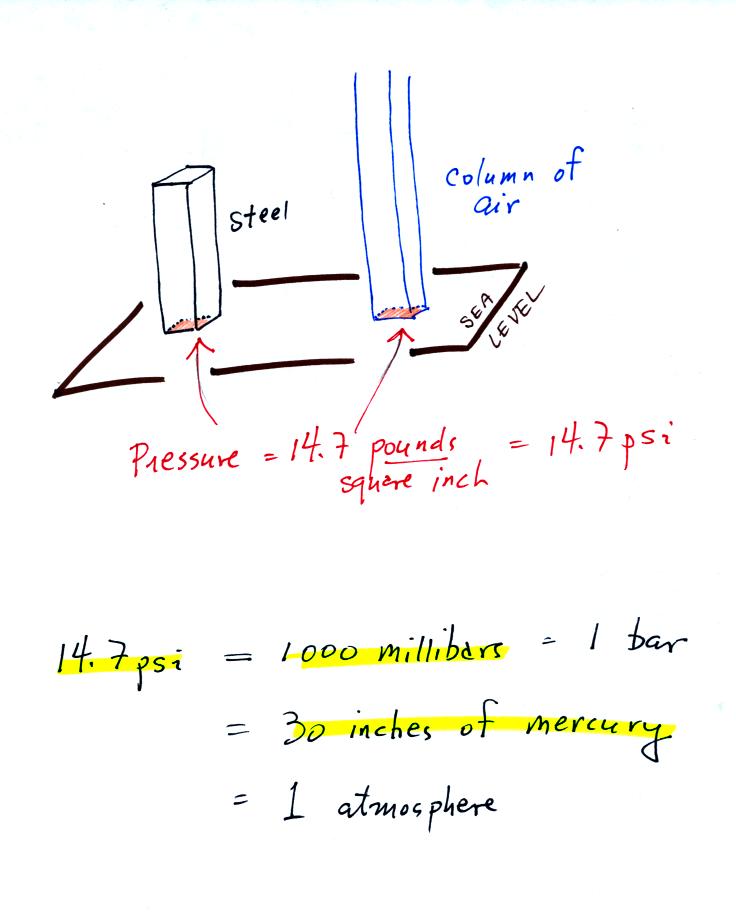
The iron bar also weighs 14.7 pounds. When it is standing on end
the bar exerts a pressure of 14.7 pounds per square inch on the ground,
the same as a 1 inch by 1 inch column of air at sea level altitude.
Some of the other commonly used pressure units are shown
above.
Typical sea level pressure is 14.7 psi or about 1000 millibars (the
units used by meterologists) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air, remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi.
The word "bar" has a lot of meanings:
An iron bar was passed around in
class. A lot of people will be watching the Super
Bowl this weekend in a bar. The word bar also refers to pressure.
Pressure
at sea level is determined by the weight of the air overhead.
What about pressure at some level above sea level?
We can use the stack of bricks sketched below to try to answer this
question.
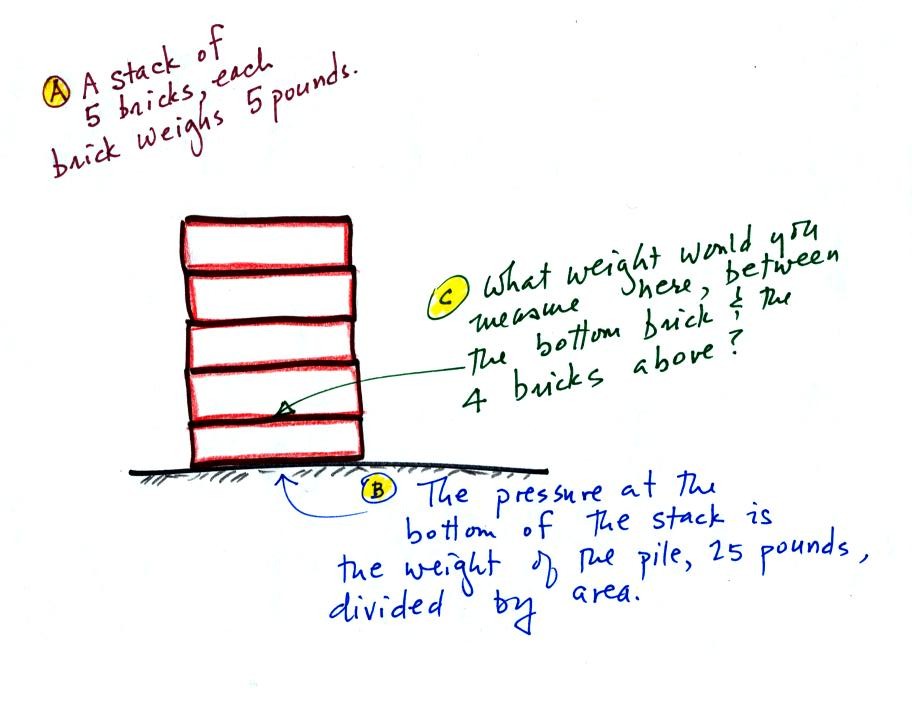
At the bottom of the pile you would measure a weight of 25 pounds (5
bricks x 5 pounds per brick). If you moved up a brick you would
measure a weight of 20 pounds, the weight of the four bricks still
above. In the atmosphere pressure at any level is determined by
the weight of the air still overhead. Pressure decreases with
increasing altitude because there is less and less air remaining
overhead.
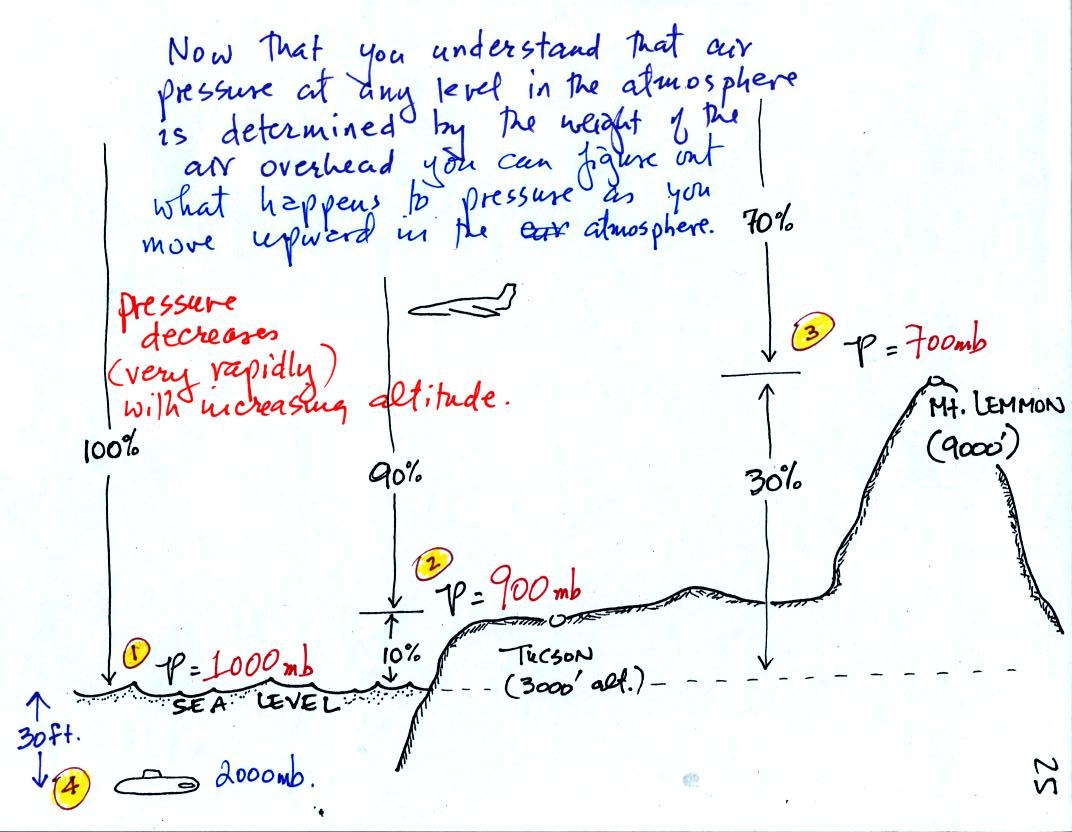 At sea level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all of the air in the atmosphere.
At sea level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000 feet above sea level (most
of the valley is lower than that). At 3000 ft. 10% of the air is
below, 90% is still overhead. It is the weight of the 90% that is
still above that determines the atmospheric pressure in Tucson.
If 100% of the atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at the summit of Mt. Lemmon (9000
ft. altitude at Point 3).
Pressure decreases rapidly with increasing altitude.
Point 4 shows a submarine at a depth of about 30 ft. The pressure
there is determined by the weight of the air and the weight of the
water overhead. Water is much denser and much heavier than
air. At 30 ft., the pressure is already twice what it would be at
the surface of the ocean.
Now
we'll take this a step further and learn that the rate of pressure
decrease with increasing altitude depends on the air density.
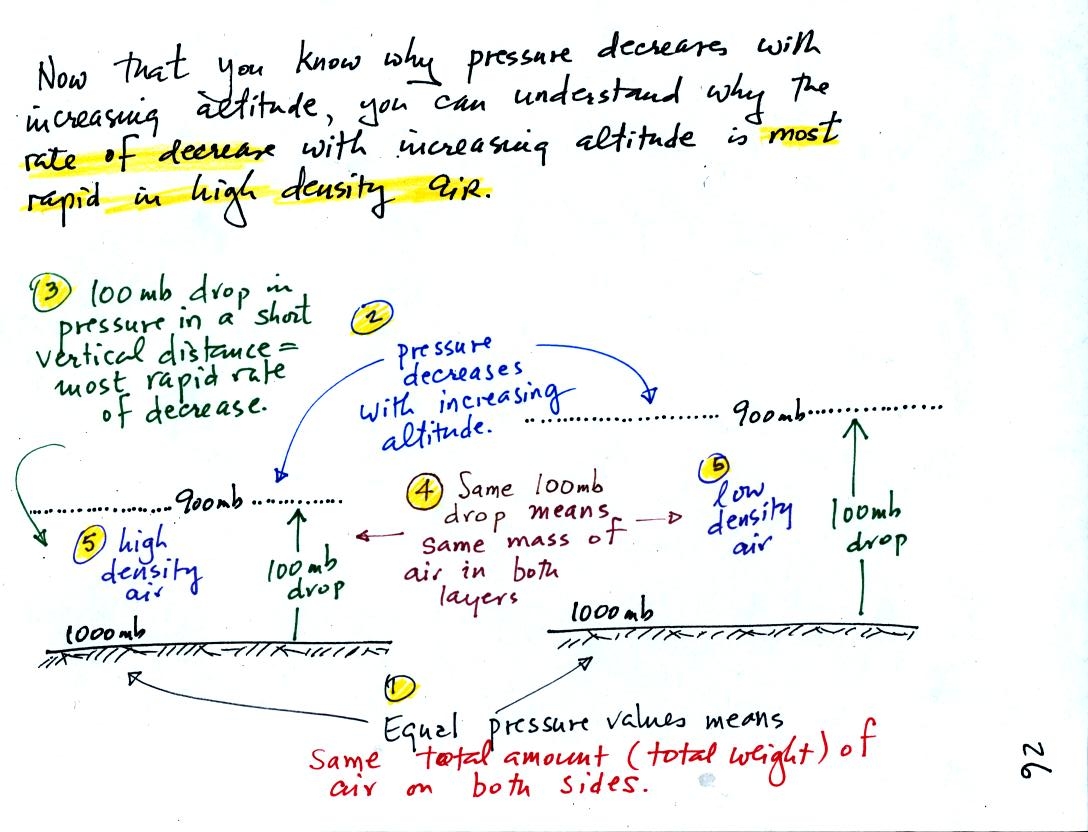
1000 mb at Point 1 is a
reasonable value for sea level pressure. The fact that the
pressures are equal at the bottoms of both
sides of the picture means that the weight of the atmosphere at the
bottom of the
picture on the left is the same as the weight of the atmosphere at the
bottom of the picture at right. The only way this can be true is
if there is the same total amount (mass) of air in both cases.
Point 2 - Moving upward from the ground we find that pressure decreases
to 900 mb at the level of the dotted line in the picture at left.
This is what you expect, pressure decreases with increasing
altitude.
Point 3 - The most rapid rate of pressure decrease with increasing
altitude is occurring in the picture at left.
Point 4 - Since there is a 100 mb drop in both the layer at left and in
the
layer at right, both layers must contain the same amount (mass) of air.
Point 5 - The air in the picture at left is squeezed into a thinner
layer than in the picture at right. The air density in the left
layer is higher than in the layer at right.
We used and analyzed this picture to prove to ourselves that the rate of pressure decrease
with altitude is higher in dense air than in lower
density air.
This is a fairly subtle but important concept. We will use this
concept several times during the semester. In particular we will
need this concept to understand why hurricanes can intensify as they do.














 At sea level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all of the air in the atmosphere.
At sea level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all of the air in the atmosphere. 