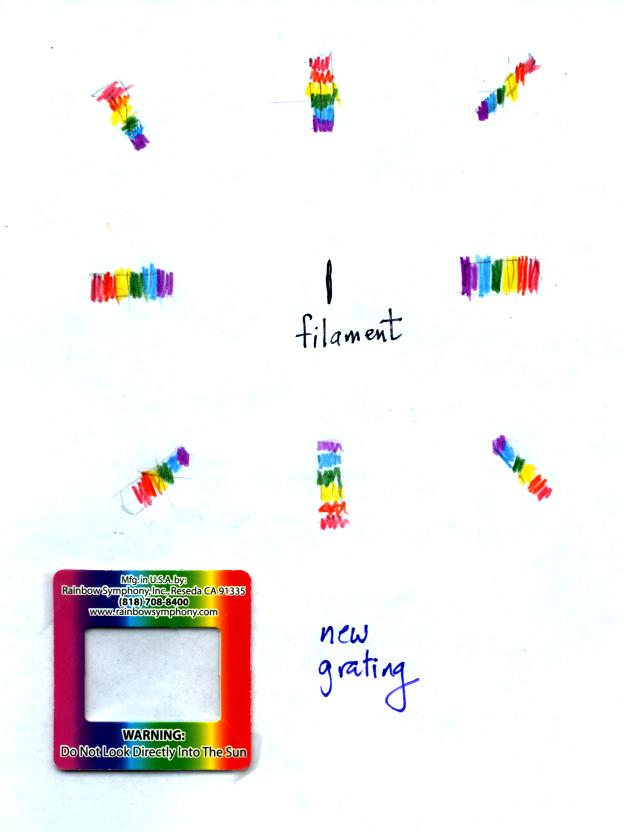Wednesday Mar. 5, 2008
The Quiz #2 Study Guide is now
available.
The Experiment #3 materials were
distributed today. You will have another opportunity to check out
materials on Friday.
The Experiment #4 materials should
become available next week.
Note: By the end of next
week you should either already have completed an experiment, should be
working on an experiment, or should be working on or have plans to
start working on one of the other options (book report, scientific
paper report).
Class
began with a quick review of the two rules governing the amount of
radiation (the Stefan-Boltzmann law) and the kind of EM radiation
(Wien's Law).

A couple of new figures that illustrate these two laws were added at
the end of the notes from Monday, Mar. 3. The graph
below also helps to illustrate the
Stefan-Boltzmann
law and Wien's law, particularly the meaning of lambda max.

Notice first that both and warm and the cold objects
emit
radiation
over a range of wavelengths. In some respects this is like quiz
grades. Not everyone in the room gets the same score; there's
usually a very wide range of scores. Objects do emit more of one
particular wavelength than any other wavelength. This is lambda
max.
The area under the warm object curve is much bigger than the area under
the cold object curve. The area under the curve is a measure of
the total radiant energy emitted by the object. This illustrates
the fact that warmer objects emit a lot more radiant energy than colder
objects.
Lambda max has shifted toward shorter wavelengths for the warmer
object. This is Wien's law in action. The warmer object is
emitting a lot of short wavelength radiation that the colder object
doesn't emit.
A bulb
connected
to a dimmer switch can be used to demonstrate the
rules above (see p. 66 in the photocopied Classnotes). We'll be
interested in and looking at the EM
radiation emitted by
the tungsten filament in the bulb.

The bottom of p. 66 has been redrawn below for clarity:
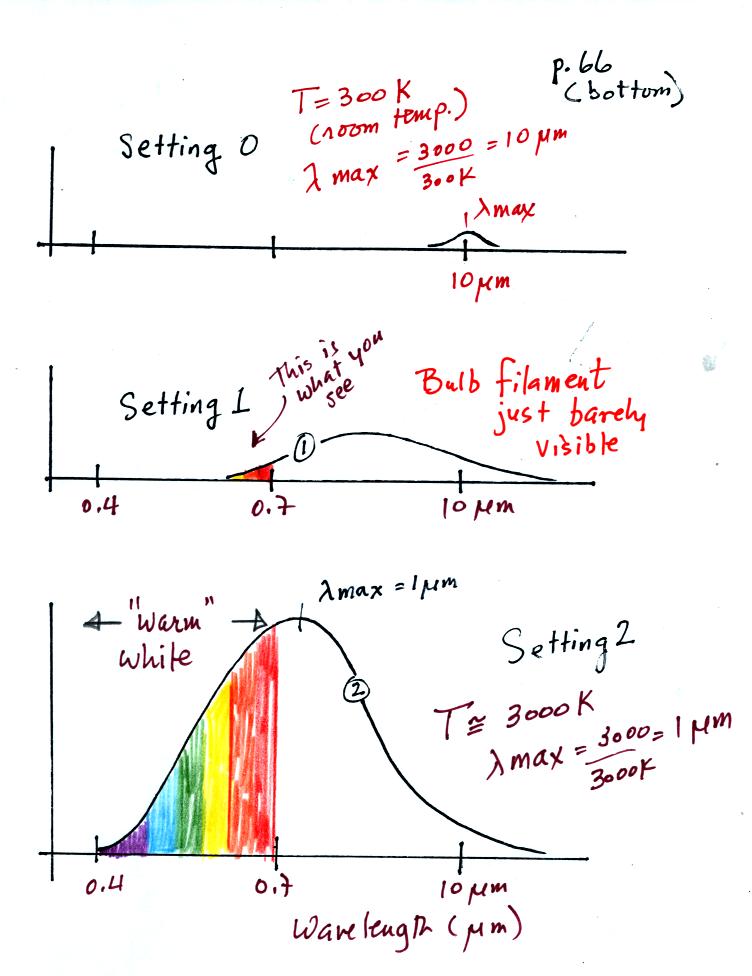
We start with the bulb turned off (Setting 0). The
filament will be at room temperature which we will assume is around 300
K (remember that is a reasonable and easy to remember value for the
average temperature of the earth's surface). The bulb will be
emitting radiation, it's shown on the top graph above. The
radiation is very weak so we
can't
feel it. It is also long wavelength far IR radiation so we
can't see it. The wavelength of peak emission is 10 micrometers.
Next we use the dimmer switch to just barely turn the bulb on (the
temperature of the filament is now about 900 K).
The bulb just barely became visible and and had an orange color.
This
is curve 1, the middle figure. Note the far left end of the
emission curve has
moved to the left of the 0.7 micrometer mark - into the visible portion
of the
spectrum. That is what you are able to see, the small
fraction of
the radiation emitted by the bulb that is visible light (but just
long wavelength red and orange light). Most of the radiation
emitted by the bulb is to the right of the 0.7 micrometer mark and is
invisible IR radiation (it is strong enough now that you could feel it
if you put your hand next to the bulb).
Finally we turn on the bulb completely (it was a 200 Watt bulb so it
got
pretty bright). The filament temperature
is now about 3000K. The bulb is emitting a lot more visible
light, all the colors, though not all in equal amounts. The
mixture of the colors produces a warm
white light. It is warm because it is a mixture that contains a
lot more red, orange, and yellow than blue, green, and violet
light. It is interesting that most of the radiation emitted by
the bulb is still in the IR portion of the spectrum (lambda max is 1
micrometer). This is
invisible light. A tungsten bulb like this is not especially
efficient, at least not as a source of visible light.
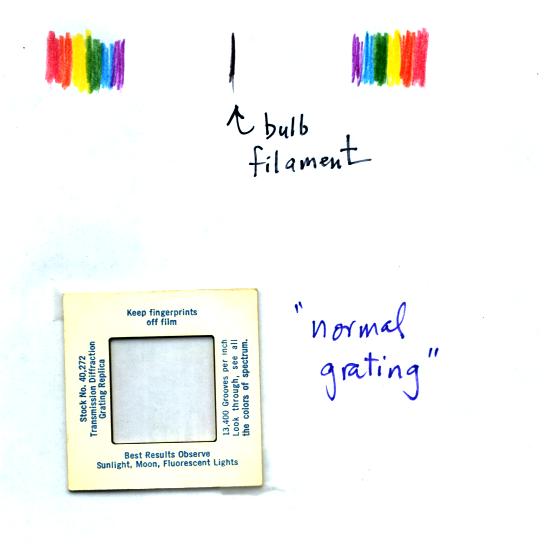
You were able to use one of the diffraction gratings to view all the
colors that make up visible light.
When you looked at the bright white bulb filament through one of the
diffraction gratings the colors were smeared out to the right and left
as shown below:
Some of the gratings behaved a little differently as shown below:
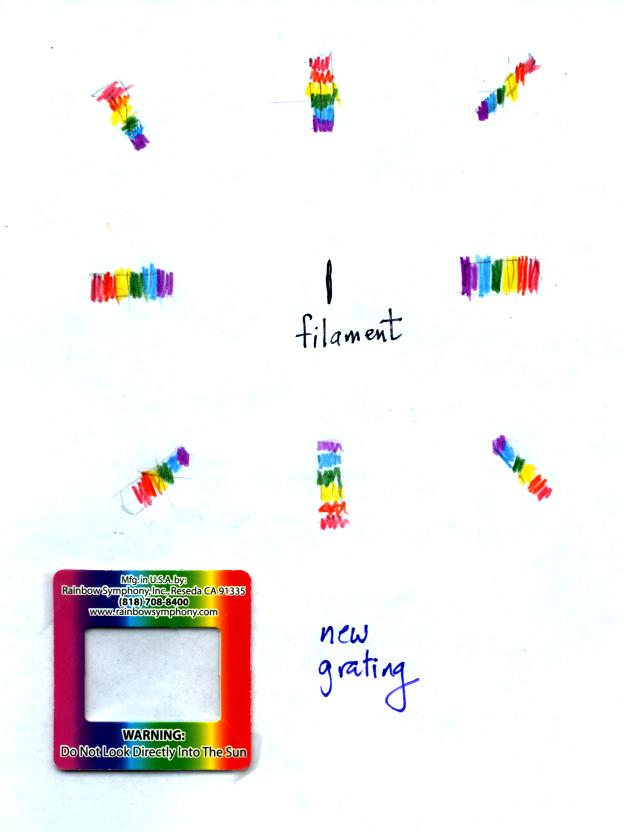
The sun
emits electromagnetic radiation. That shouldn't come as a surprise
since you can see it and feel it. The earth also emits
electromagnetic radiation. It is much weaker and invisible.
The kind and amount of EM radiation emitted by the earth and sun depend
on their respective temperatures.
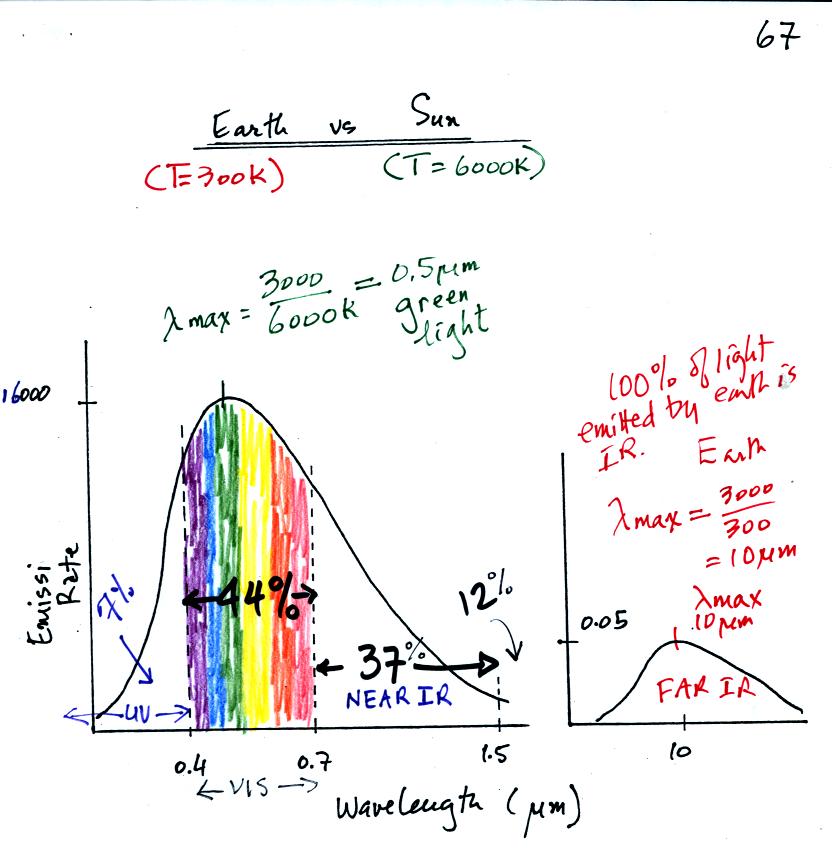
The curve on the left is for the sun. We first used Wien's
law and a temperature of 6000 K to calculate lambda max and got
0.5 micrometers. This is green light; the sun emits more green
light than any other kind of
light. The sun doesn't appear green because it is also emitting
lesser amounts of violet, blue, yellow, orange, and red - together this
mix of
colors appears white. 44% of the radiation emitted by the sun is
visible light, 49% is IR light (37% near IR + 12% far IR), and 7%
is ultraviolet light. More than half of the light emitted by the
sun is invisible. We can't see it, but we can feel it.
100% of the light emitted by the earth (temperature = 300 K) is
invisible IR light. The
wavelength of peak emission for the earth is 10 micrometers.
Because the sun (surface of the
sun) is 20 times hotter than the earth a square foot of the sun's
surface emits energy
at a rate that is 160,000 (20x20x20x20) times higher than a
square foot on the
earth. Note
the vertical scale on the earth curve is different than on the sun
graph. If both the earth and sun were plotted with the same
vertical scale, the earth curve would be too small to be seen.
We saw
earlier that tungsten bulbs produce a lot of wasted infrared light
(wasted in terms of not lighting up a room). They also produce a
warm white color. Energy efficient compact
fluorescent lamps (CFLs) are
designed to mimic the visible light output of a tungsten bulb without
producing a lot of wasted infrared light. CFLs come with
different color temperature ratings.
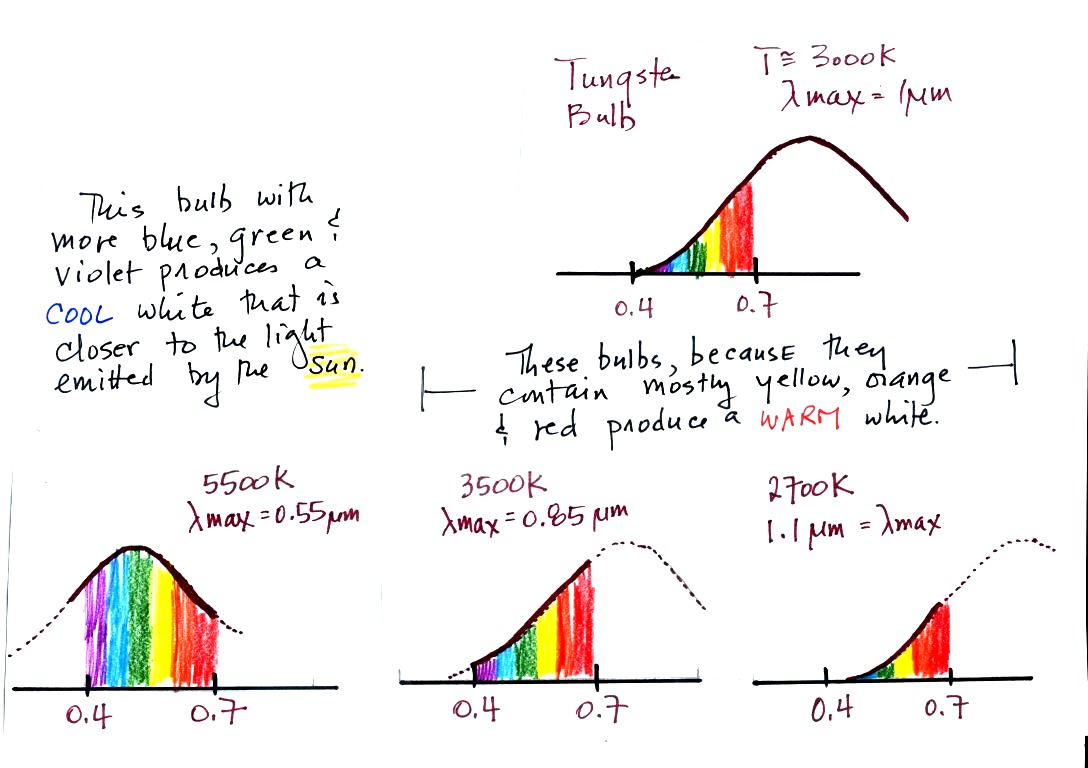
The bulbs with the hottest temperature rating (5500 K ) in
the figure above emits more purples, blues, and greens and produces a
cooler, bluish white. This is much closer to the light emitted by
the sun.
The tungsten bulb (3000 K) and the CFLs with temperature ratings of
3500 K and 2700 K produce a warmer white.
Three CFLs with the temperature ratings above were set up in class so
that you could see the difference between warm and cool white light.
You can see a clear difference between the cool white bulb on the left
in the figure below and the warm white light produced by a tungsten
bulb (2nd from the left) and 2 CFCs with low temperature ratings (3rd
and 4th from the left). This figure is from an article
on compact fluorescent lamps in Wikipedia.

We now
have most of the tools we will need to begin to study energy balance on
the earth. It will be a balance between incoming sunlight
energy and outgoing energy emitted by the earth. We will look at
the simplest case, first, the earth without an atmosphere (or at least
an atmosphere without greenhouse gases) found on p. 68 in the
photocopied Classnotes.
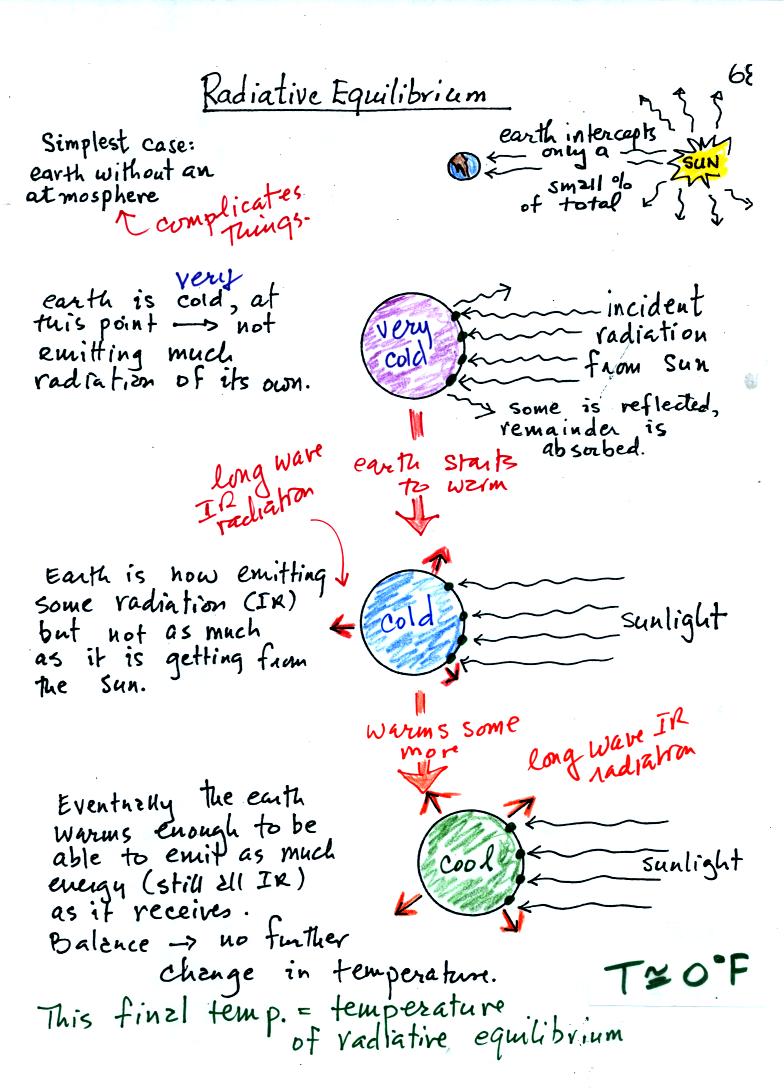
You might first wonder how, with the sun emitting so much
more
energy than the earth, it is possible for the earth to be in energy
balance with the sun. The earth is located about 90 million miles
from the sun and therefore only absorbs a very small fraction of the
energy emitted by the sun.
To understand how energy balance occurs we start, in Step #1, by
imagining that the earth starts out very cold and is
not emitting
any EM radiation at all. It is absorbing sunlight however so it
will
begin to warm.
Once the earth starts to warm it will also begin to emit EM
radiation, though not as much as it is getting from the sun (the
slightly warmer earth in the middle picture is now colored blue).
Because the earth is still gaining more energy than it is losing the
earth will warm some more.
Eventually it will warm enough that the earth (now shaded green) will
emit the same amount
of energy (though not the same wavelength energy) as it absorbs from
the sun. This is radiative equilibrium, energy balance. The
temperature at
which this occurs is about 0 F.
That is called the temperature of radiative equilibrium. You
might remember this is the figure for global annual average surface
temperature on the earth without the greenhouse effect.
The section on the filtering effect of
the atmosphere on light has been moved to the Friday, Mar. 7
notes. We will start class on Friday by quickly reviewing that
material.