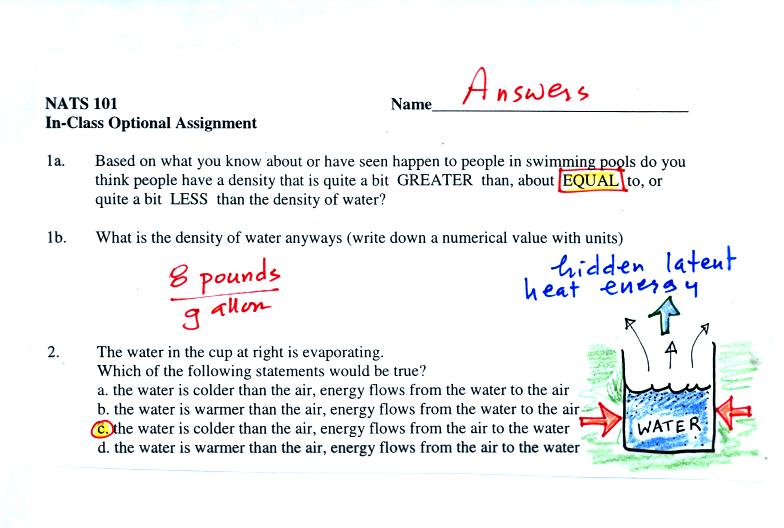
The evaporating water in the glass is taking energy from the water
remaining in the glass. This will cause the water to cool (just
as you cool when you step out of a shower and are still wet).
Once the water becomes cooler than the surrounding air, energy will
flow from the air into the glass.
This is a question that would get me into trouble on a quiz. Many
people probably recognized that the movement of water vapor from the
glass out into the surrounding air is latent heat energy
transport. What is ultimately happening is that heat the first
flowed from the room into the glass of water is "stored" in the water
vapor. This energy would reappear whenever the water vapor
condensed.

Students in the T Th class didn't get a chance to see the sublimating
iodine. They can go to the WebElements
site and see the solid and gaseous iodine (the iodine gas has a
pinkish-purple color).
In Question 4 you could see the cloud that formed around the cold dry
ice. Moist air in contact with the dry ice was cooled to its dew
point. As the water vapor condenses it releases latent heat
energy that flows into the dry ice. The cloud also made the
sinking air motions visible. Sinking air is free
convection. Warm air coming into contact with the dry ice gives
up energy and becomes cold high-density air. As the cold air
sinks it gets out of the way and is replaced by warm air which thens
gives up its energy and the cycle repeats itself.

Heat will flow the warm air into the cold cans at the same rate.
The water vapor that is condensing onto Can B is an additional source
of energy. Energy is given up as water vapor condenses.


