We listed
the 5 most abundant gases in the atmosphere in class on Monday.
Here is a list of several more important gases.
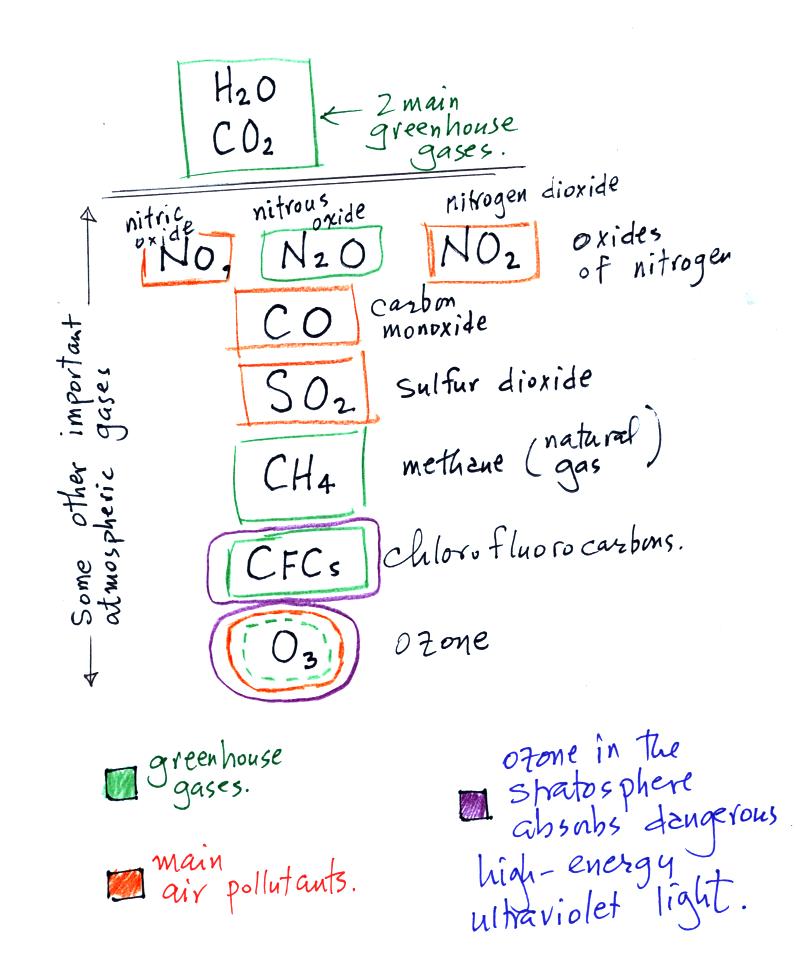
Water vapor, carbon dioxide, methane, nitrous oxide (N2O
=
laughing gas),
chlorofluorocarbons, and ozone are greenhouse gases. We'll
discuss the greenhouse effect a little bit more later in class today
and will
learn more about it actually works when we get to Chapter
2.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants. We'll cover 2 or 3
of these in class on Friday and early next week. You may have
heard or read about an incident earlier this week where carbon monoxide
from a malfunctioning hot water heater sickened 23 Virginia Tech
students in an apartment complex. Carbon monoxide
concentrations indoors can easily and rapidly reach fatal levels.
Carbon monoxide
levels in the atmosphere are much lower but can still represent a
health hazard.
Ozone in the stratosphere (a layer of the atmosphere between 10 and 50
km) is beneficial because it absorbs dangerous high energy ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone. In the
troposphere (the bottom 10 kilometers of the atmosphere) ozone is a
pollutant and is one of the main ingredients in photochemical smog.
