At this
point we made a not so smooth transition to carbon monoxide, an
important air pollutant. We'll finish up CO in class on Monday
and also cover ozone.
Some basic information about carbon monoxide is shown below (p. 7 in
the photocopied Class Notes). You'll find
additional information at the Pima
County Department of
Environmental Quality website and also at the US Environmental Protection Agency
website.

Carbon monoxide molecules bond strongly to the hemoglobin
molecules in
blood and interfere with the transport of oxygen through your
body. CO is a primary pollutant. That means it goes
directly from a source into the air (nitric oxide, NO, and sulfur
dioxide, SO2, are also primary pollutants). CO is
emitted directly from an automobile tailpipe into the atmosphere for
example
CO is produced by incomplete combustion of fossil
fuel. Complete combustion would produce carbon dioxide,
CO2. Cars and trucks produce much of the CO in
the
atmosphere. Vehicles must now be fitted with a catalytic
converter which will change CO into CO2 (and also NO into N2
and
O2). In Pima County vehicles must pass an emissions
test every
year and special formulations of gasoline (oxygenated fuels) are used
during the winter months to try to reduce CO emissions. See if
you can figure out why carbon monoxide is often a problem in cities at
high altitude (the answer is found at the bottom of today's online
notes)
Carbon monoxide is also a serious hazard indoors.
Because it is odorless, concentrations can build to dangerous levels
without you being aware of it. You can purchase a carbon monoxide
alarm that will monitor CO concentrations indoors and warn you when
concentrations reach hazardous levels. Indoors CO is
produced by gas furnaces and water heaters that are
either operating improperly or aren't being adequately vented
outdoors. Many people are killed indoors by carbon monoxide every
year. You can learn
more about carbon monoxide hazards and risk prevention at the Consumer Product
Safety Commission web page.
In the atmosphere CO concentrations peak on winter mornings.
Surface temperature inversion layers form on long winter night when the
ground becomes colder than the air above. Air in contact with the
cold ground cools and ends up colder than air above. Air
temperature increases with increasing altitude in a temperature
inversion and this produces a very stable layer of air at ground level.
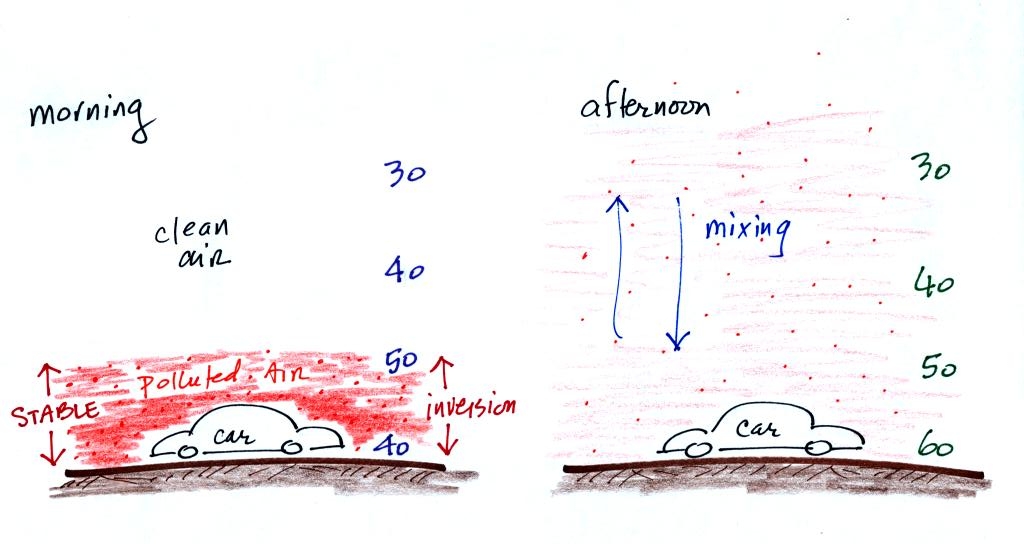
When CO is emitted into a thin stable layer (left figure above), the CO
remains in the layer and doesn't mix with cleaner air above. CO
concentrations build.
In the afternoon the atmosphere becomes more unstable. CO emitted
into air at the surface mixes with cleaner air above. The CO
concentrations are effectively diluted and don't get as high as they do
in the morning.
A portion of a time lapse cloud move was shown at the end of
class. Thunderstorms were developing over the Catalina
mountains. Thunderstorms are a visible indication of unstable
atmospheric conditions.
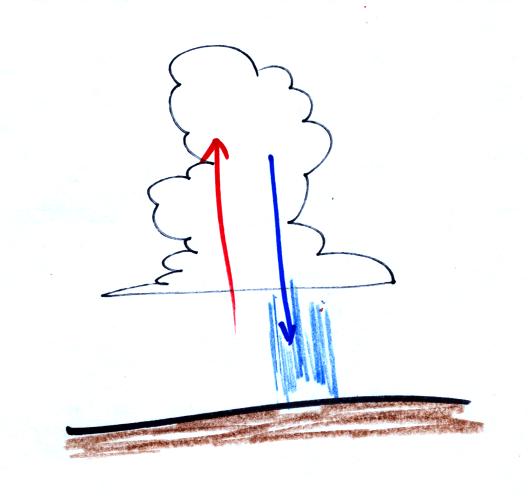
You could see the clouds growing vertically in the movie, evidence of
rising air motions. Falling precipitation also produces a
downdraft, sinking air motions. This downdraft is the source of
the strong, often damaging, surface winds that accompany thunderstorms.
We'll
finish the section on carbon monoxide today. Last week we learned
that carbon monoxide is a primary pollutant produced by incomplete
combustion. Peak CO concentrations are observed on winter
mornings.

Six main pollutants are listed at the top of this page.
Concentrations of some or all of these pollutants are measured daily in
many
cities. The atmospheric concentration of lead has decreased
significantly since the introduction of unleaded gasoline. PM
stands for particulate matter. These small particles are
invisible, remain suspended in the air, and may be made of harmful
materials..
CO, O3 and particulate matter are the pollutants of most
concern in
Tucson and pollutant concentrations are reported in the newspaper or on
television using the Air Quality Index (formerly the pollutant
standards index). This is basically the measured value divided by
the allowed value multiplied by 100%. Current Air Quality Index values for
Tucson are available online.

The first graphs shows a typical atmospheric temperature profile
near the ground in the winter. The inversion is the bottom
portion of the plot where temperature increases from 47 F to near
60 F with 1000 feet of altitude gain. The 1000 foot deep
layer is a stable layer.
The middle figure shows some of the health effets and symptoms of CO
poisoning. The effect of CO depends on both the concentration and
the length of exposure. The NAAQS values are shown at
bottom of the chart. Exposure to CO concentrations of these
levels shouldn't cause any symptons in a healthy individual.
Concentrations reached 500 ppm in the apartment building near the
campus of Virginia Tech. Several students were unconscious when
found by rescue personnel.
The bottom figure shows average monthly AQI values for CO and O3
in
Tucson. CO concentrations (blue curve) tend to peak on winter
mornings.
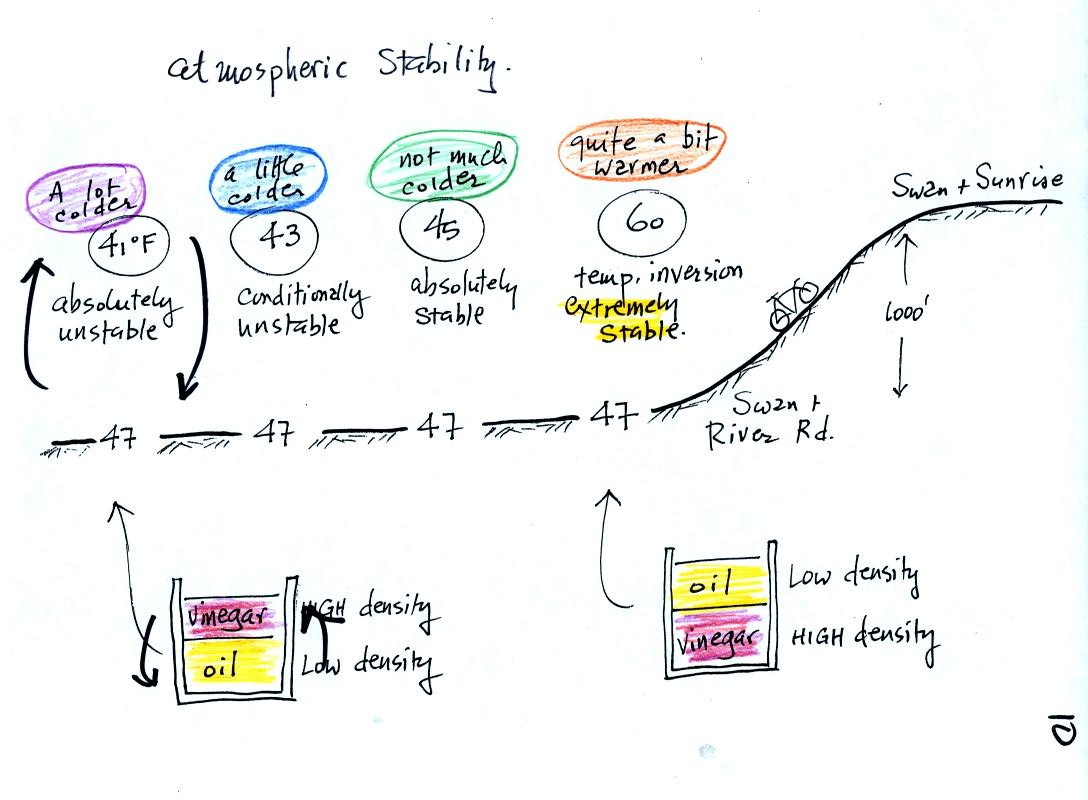
This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for the
purposes of illustration we imagine riding a bicycle from Swan and
River Rd up a hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that)
At far left the air temperature drops 6o F. This is a
fairly
rapid rate of decrease with increasing altitude and would make the
atmosphere
absolutely unstable. The atmosphere wouldn't remain this
way. Air at the ground would rise, air above would sink, and the
temperature profile would change. In some ways it would be like
trying to pour vinegar on top of oil in a glass. The lower
density oil would rise because it would "want" to float on top of the
higher density vinegar.
The next picture shows air temperature decreasing a little more slowly
with increasing altitude. This small change makes the atmosphere
conditionally unstable (we won't go into the conditions). The
atmosphere is frequently in this state.
The atmosphere cools only 2o F in the next picture.
This creates
an absolutely stable atmosphere. Air at the ground will remain at
the ground and won't rise and mix with air higher up. Compare
this with the glass containing vinegar and a layer of oil on top.
The two layers won't mix.
Air temperature in the last figure actually increases with increasing
altitude, common on winter mornings in Tucson (and worth bicycling up
the hill on Swan Rd. just to experience on a cool winter morning).
This is a temperature inversion and produces very
stable conditions. If you do find yourself on a bicycle at
Swan and Sunrise, check out the very steep section at the far northern
end of Swan.





