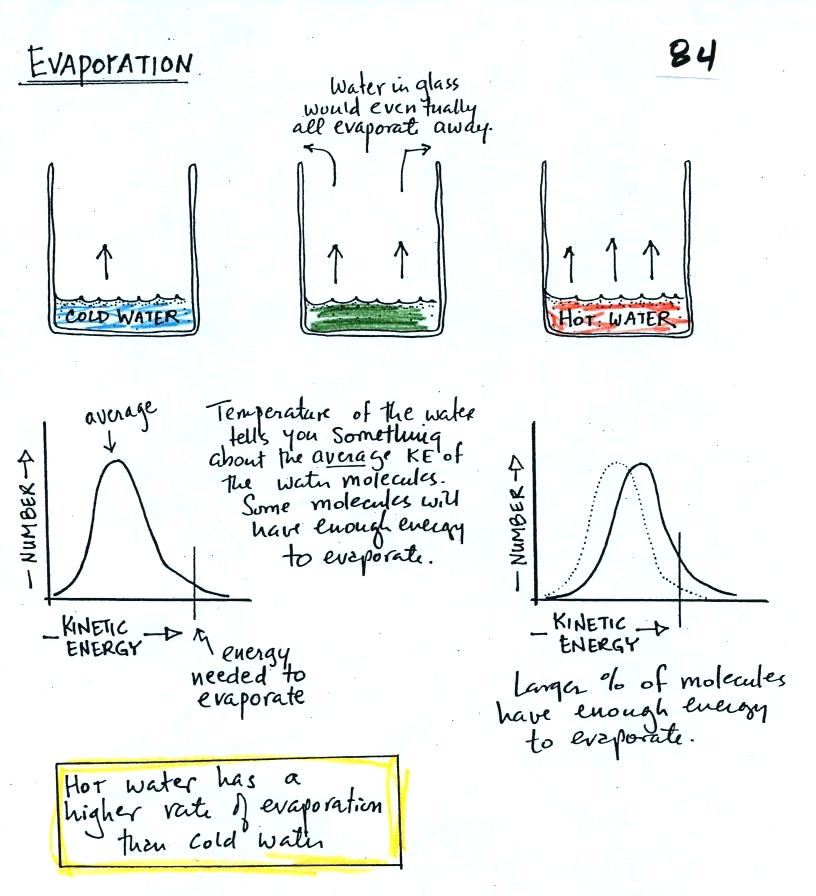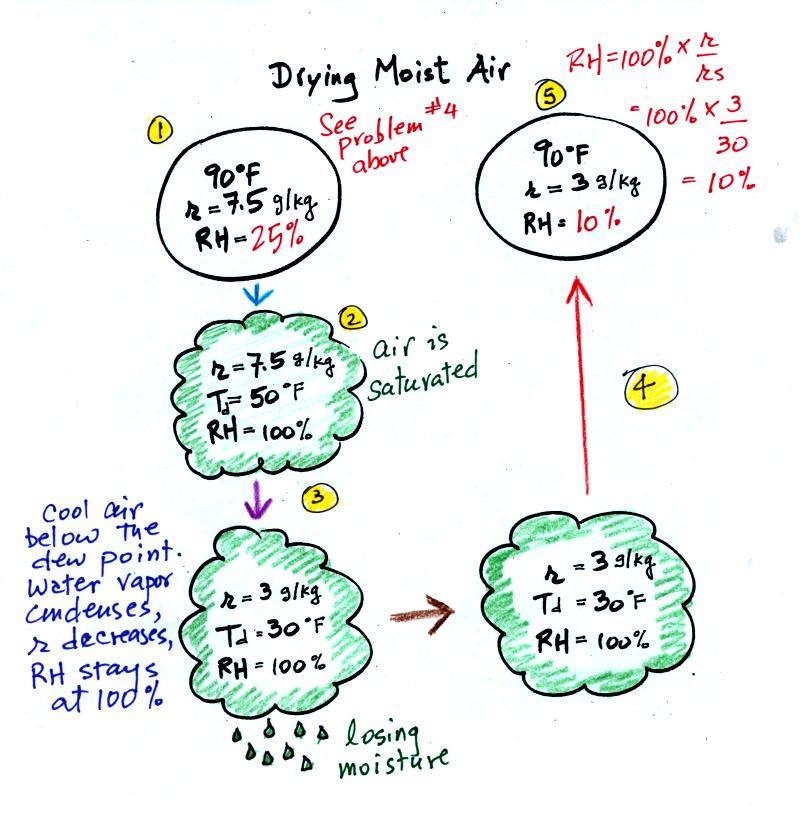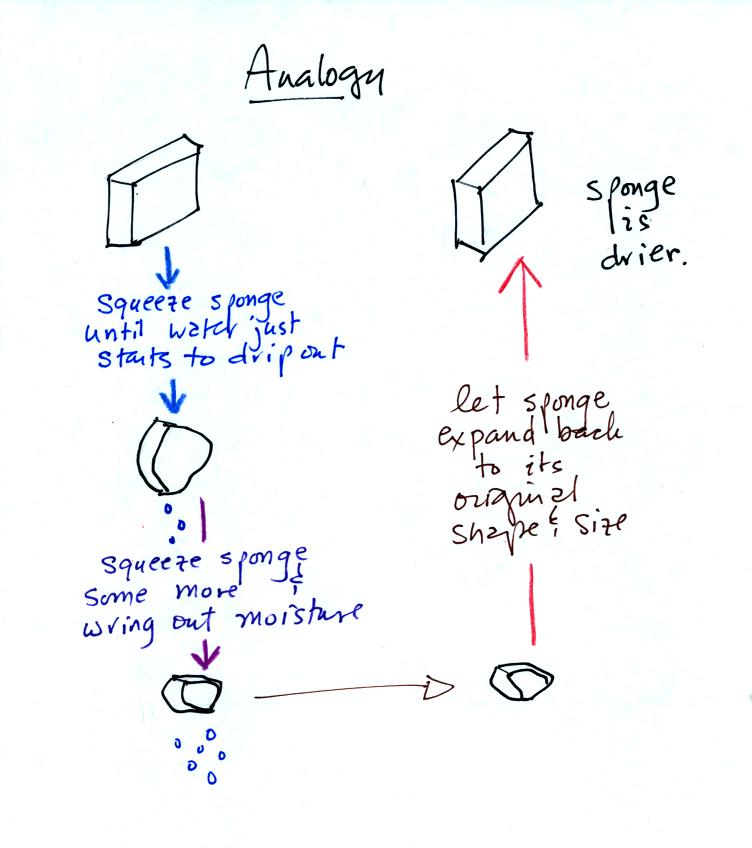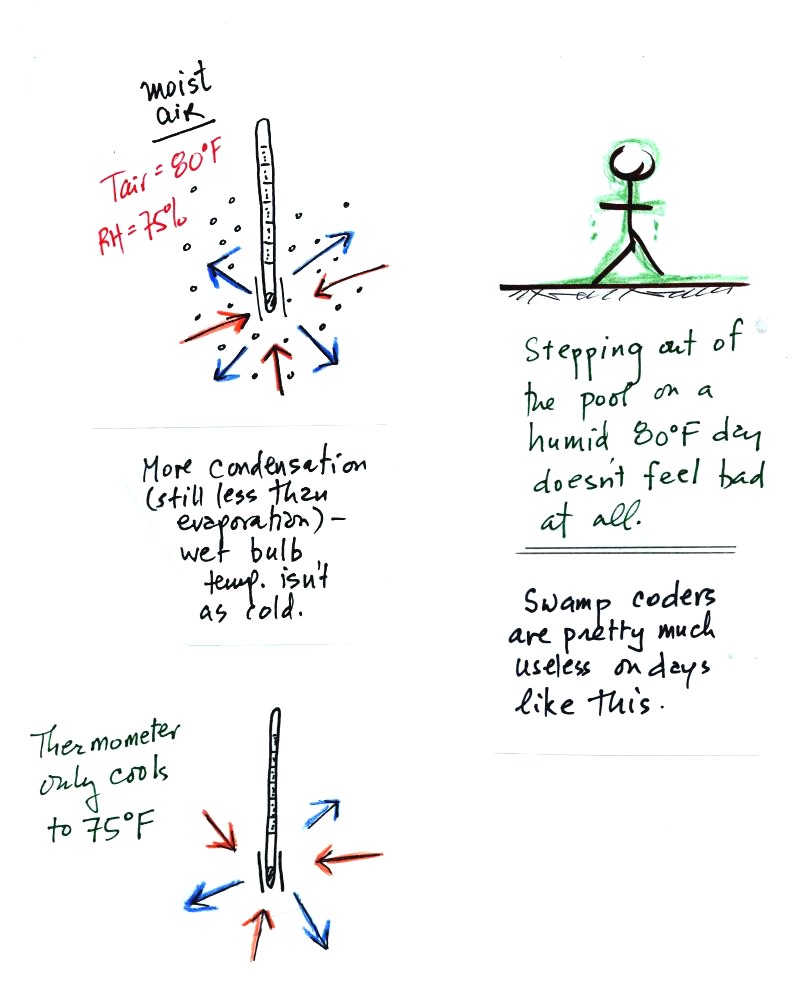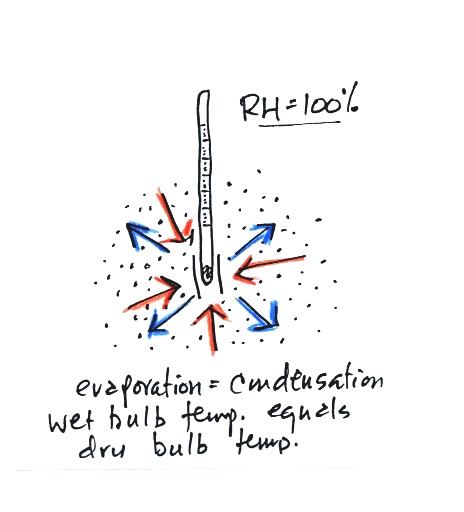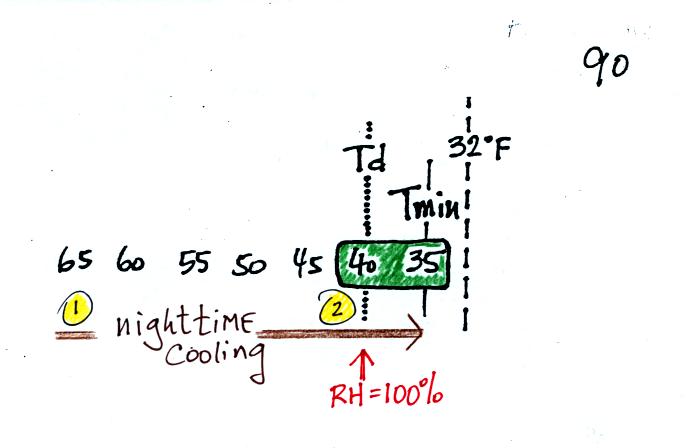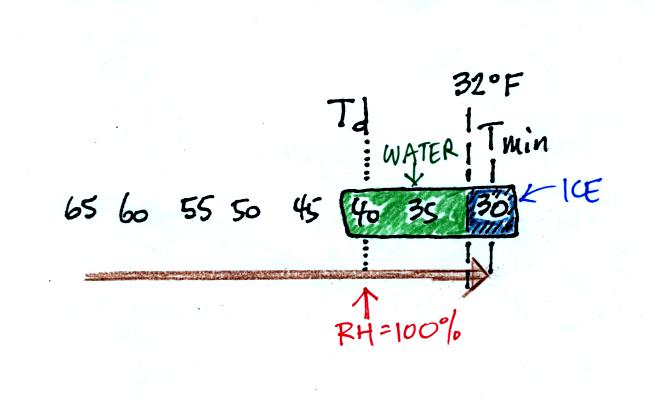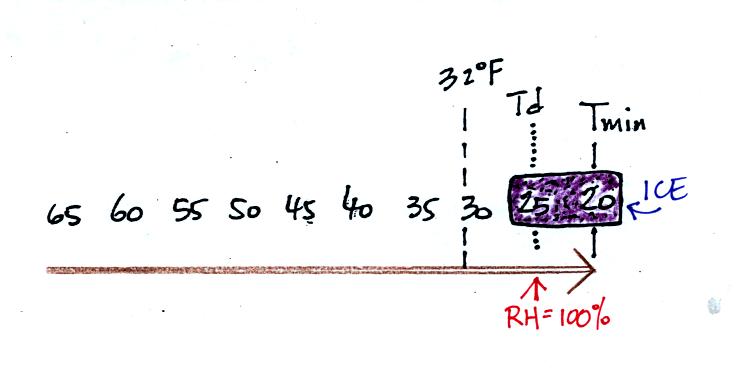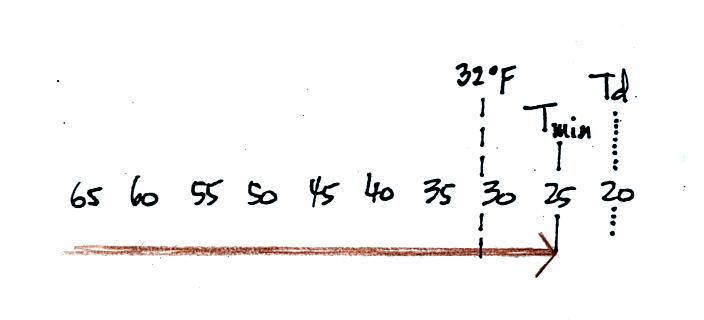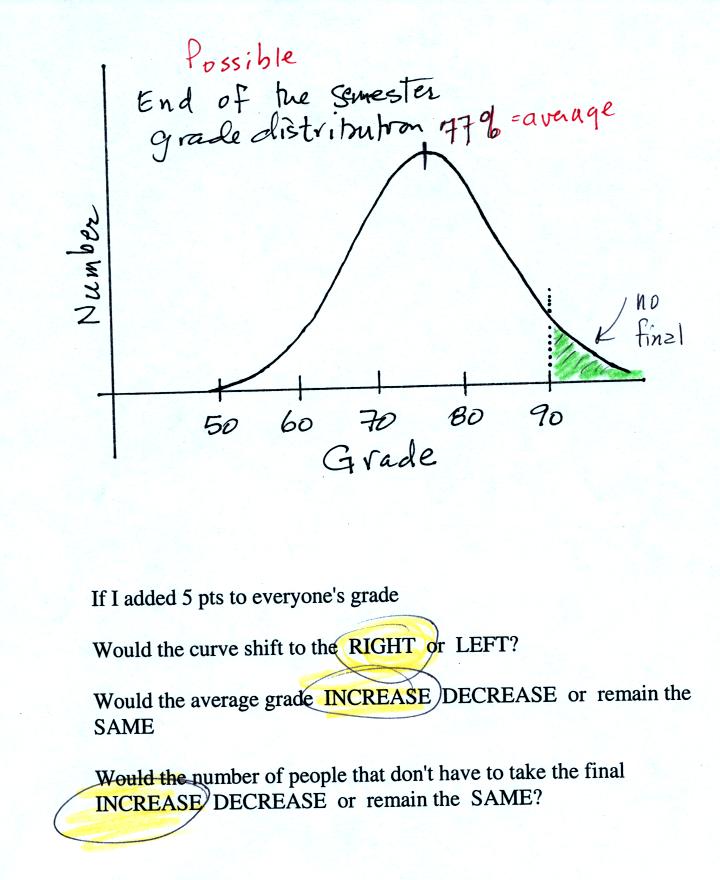
The graph shows a reasonable
distribution of end-of-semester
grades. The average appears to be about 77%. Students with
grades equal to or greater than 90.0% are exempt from the final.
A student in the class was asked the three questions at the bottom of
the figure. If 5 pts were added to everyone's overall grade, the
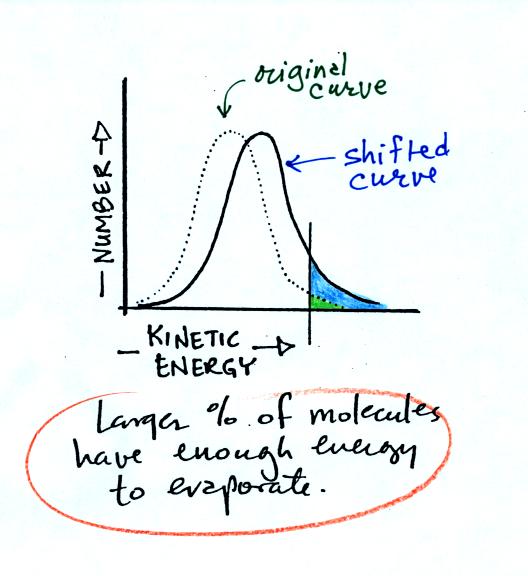
curve and the average grade would both shift to the right. This
rightward shift would increase the number of students that wouldn't
have to take the final exam.

This is the shifted curve.