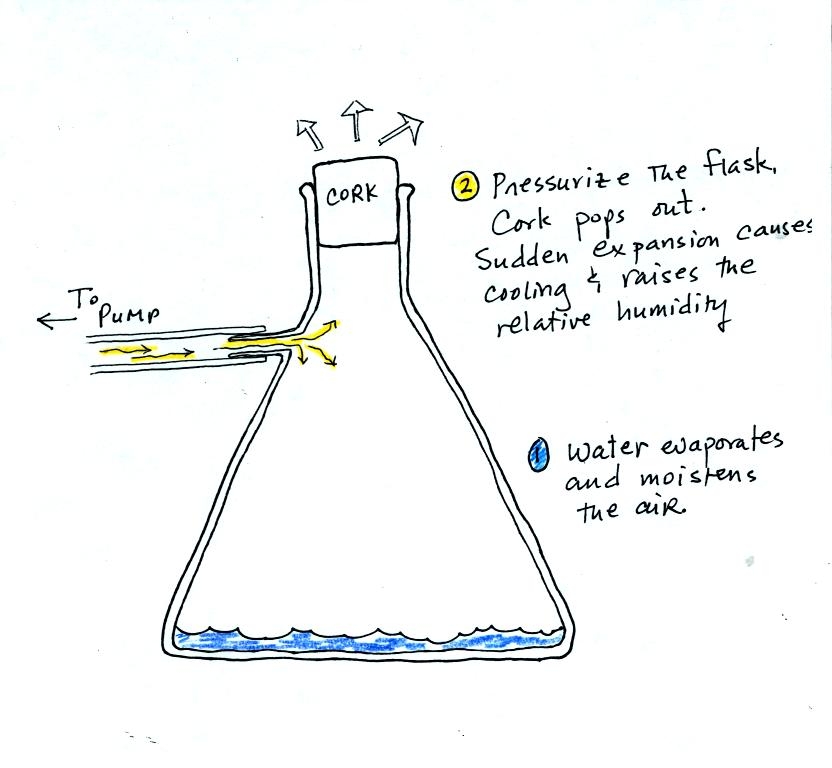
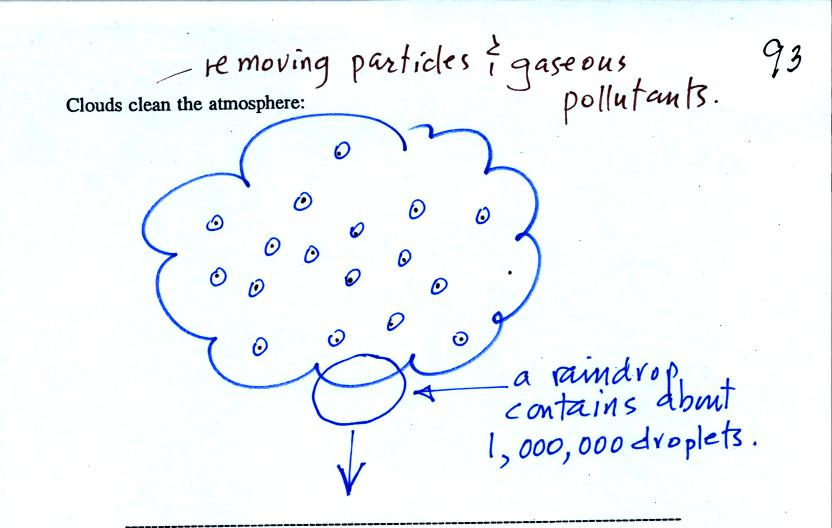
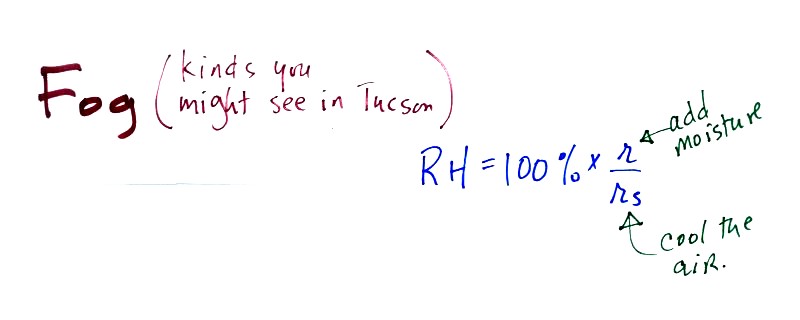You can learn why it is so hard to
form small droplets of
pure water by
reading the top of p. 92 in the
photocopied class notes.
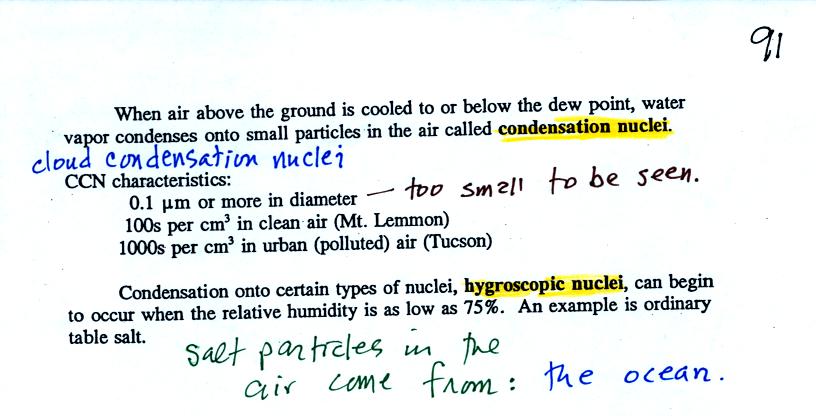
Water vapor will condense onto
certain kinds of condensation
nuclei
even when the relative humidity is below 100% (again you will find some
explanation of this on the bottom of
p.
92). These are called hygroscopic
nuclei.
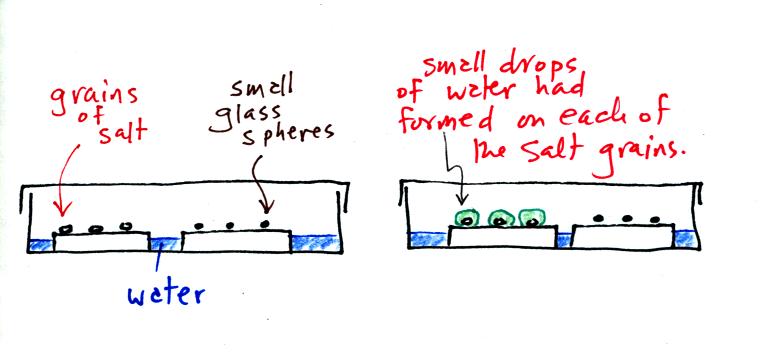
A short video showed how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of
glass. The figure below
wasn't shown in class.
The start of the video at left
showed the small grains
of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right).
In humid parts of the US, water will condense onto the grains of
salt
in a salt shaker causing them to stick together. Grains of rice
apparently absorb moisture which keeps this from happening and allows
the salt to flow
freely out of the shaker when needed.
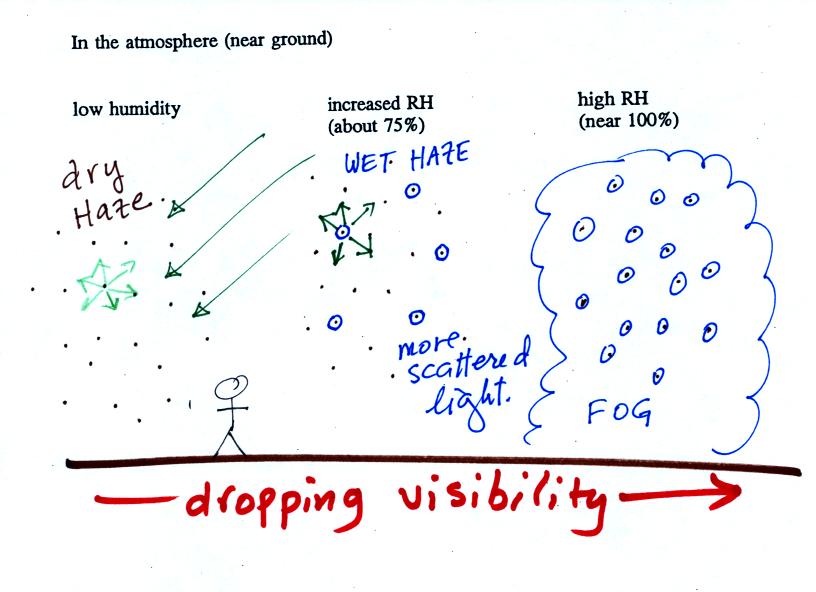
This figure (bottom of p. 91)
shows
how
cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
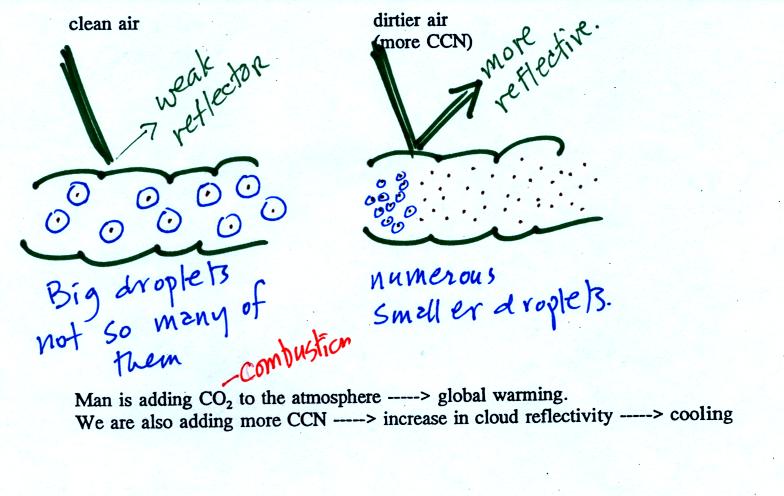
The air in the left most figure is relatively dry. Even
though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter
sunlight. When you look at the sky you see the deep blue color
caused by scattering of sunlight by air molecules mixed together with
some white
light scattered by the condensation nuclei. This changes
the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze."
The middle picture shows what happens when you drive from the dry
southwestern part of the US into the humid
southeastern US. One of the first things you would notice is the
hazier
appearance of the air and a decrease in visibility. Because the
relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze."
Finally when the relative humidity increases to 100% fog
forms.
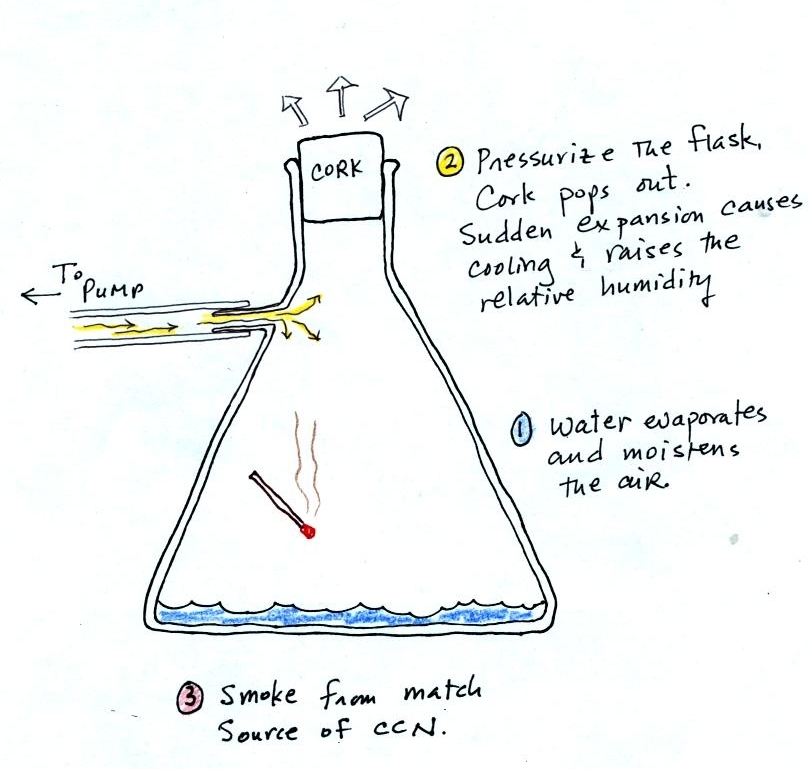
Fog can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation nuclei. We
will see this effect in the cloud-in-a-bottle demonstration coming up
at the end of class.
There are
two types of fog that you might occasionally see in
Tucson (fog is fairly infrequent because the air is so dry)
To produce fog you first need to increase the relative humidity (RH) to
100%
You can do this either by cooling the air or adding moisture to
and saturating the air (both will increase the ratio in the RH formula
above).
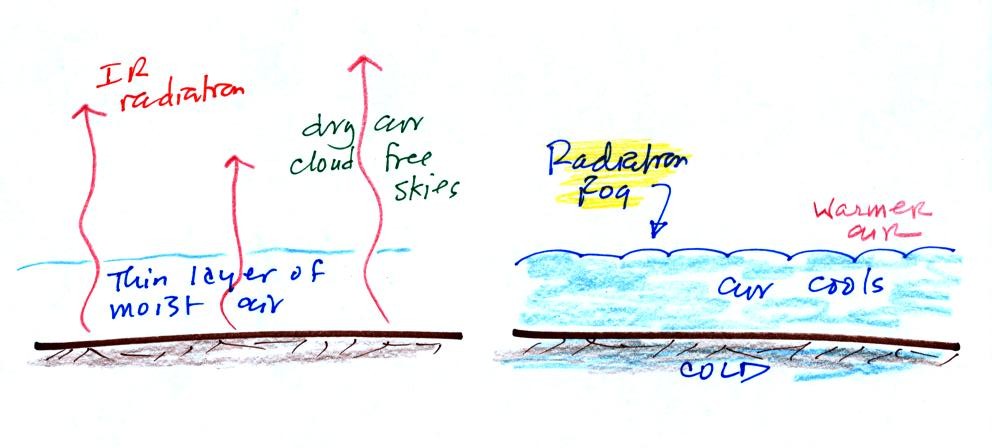
The ground cools during the night by emitting IR radiation (left figure
below). The ground cools most rapidly when the skies are free of
clouds and the air is dry (except for a thin layer next to the
ground). These are the conditions that favor the formation of
radiation fog.
Air in contact with the ground cools and radiation fog can form
(right
figure above). Because the fog cloud is colder than the air right
above, this is a stable situation. The fog clouds "hugs" the
ground.
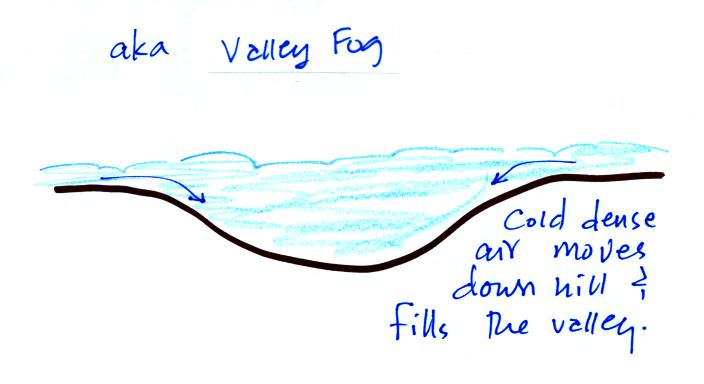
Radiation fog is sometimes called valley fog.
The cold dense air will move downhill and fill low lying areas with fog
cloud. It is often difficult for the sun to warm the air
and dissipate thick clouds of valley fog.
Steam fog (aka evaporation fog or mixing fog) is commonly observed on
cold mornings over the relatively warm water in a swimming pool.
Water evaporating from the pool
saturates the cold air above. Because the fog cloud is warmer
than the cold surrounding air, the fog clouds float upward.
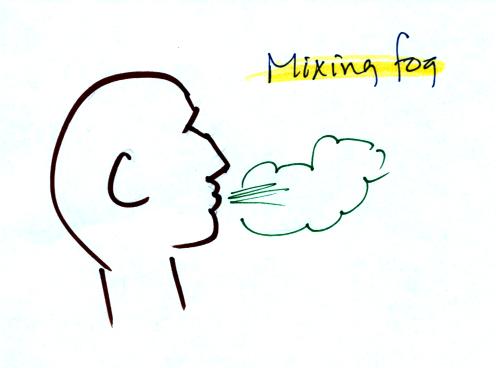
When you "see your breath" on a cold day (the figure below wasn't shown
in class)
you're seeing mixing fog. Warm moist air from your mouth mixes
with the colder air outside. The mixture is saturated and a fog
cloud forms.
If there
ever was a time for a demonstration it was class on Friday (honestly
I've rarely seen the negative vibes reach the level they did last
Friday).
Cooling
air and
changing relative humidity, condensation nuclei, and scattering of
light are all involved in this demonstration.