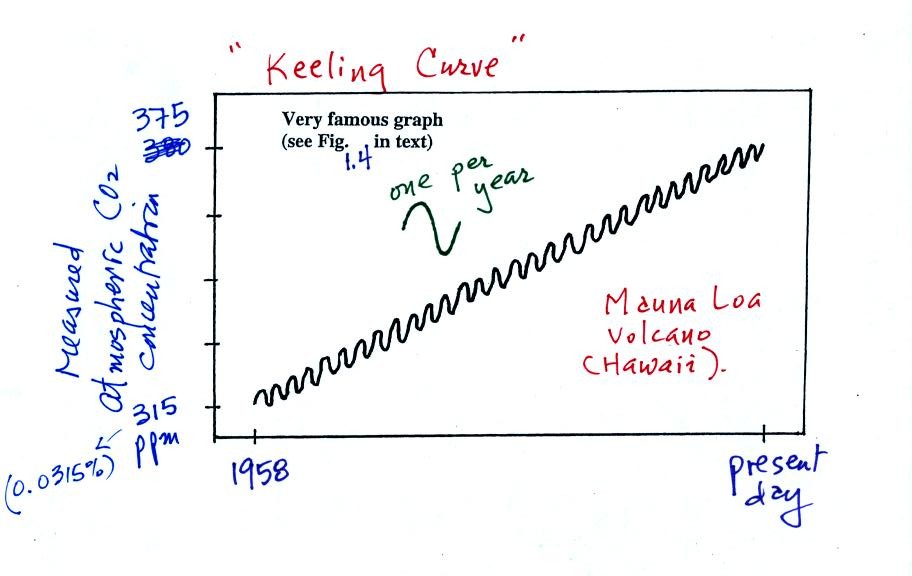
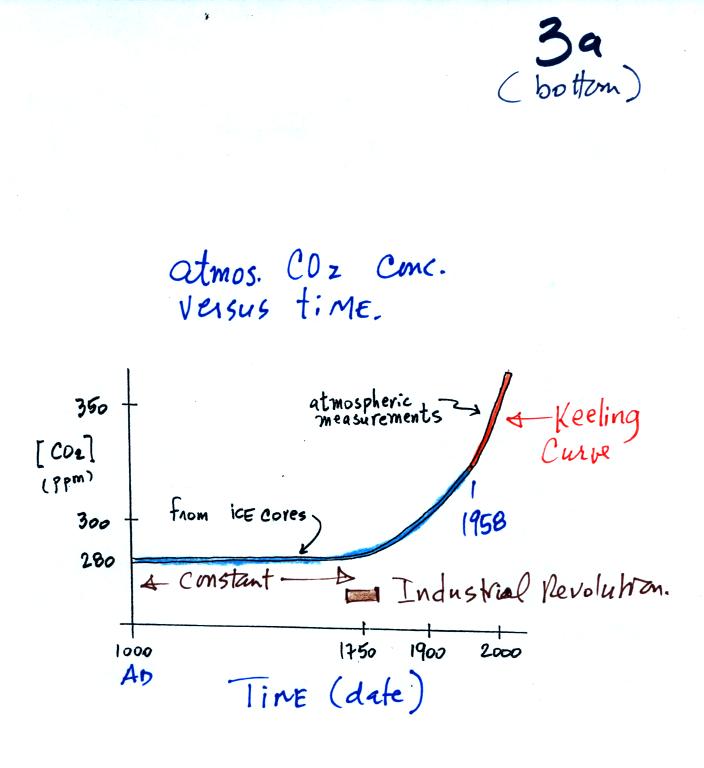
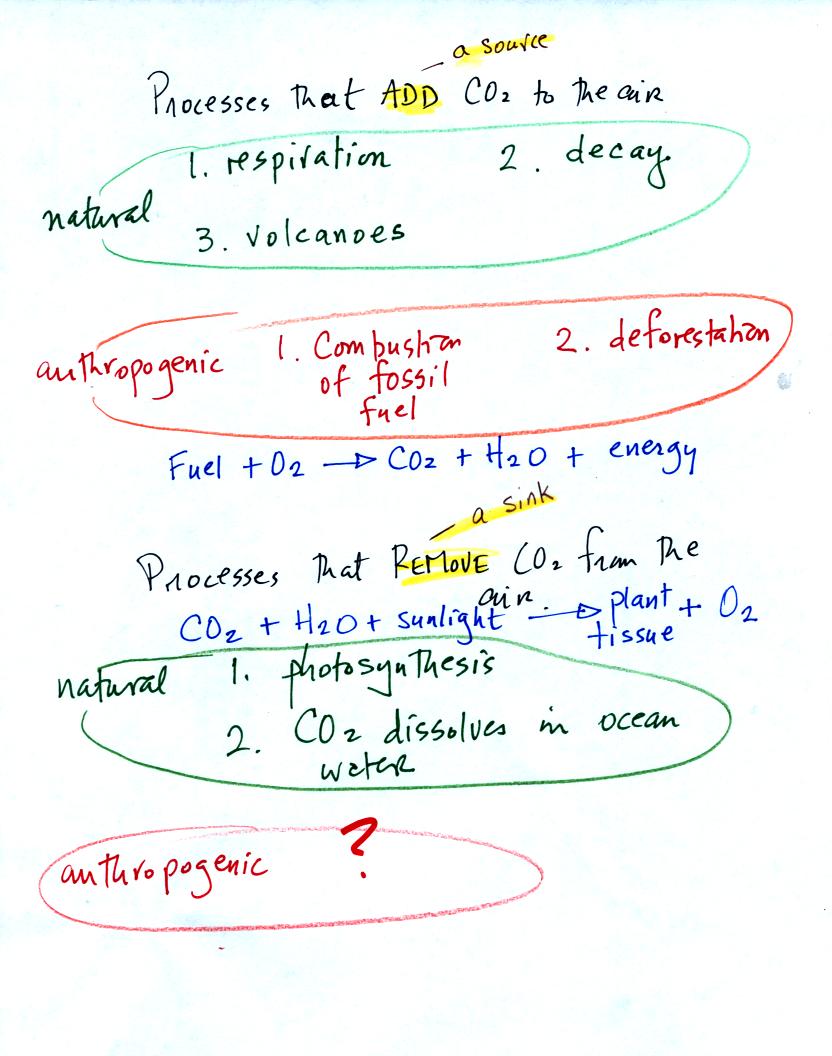
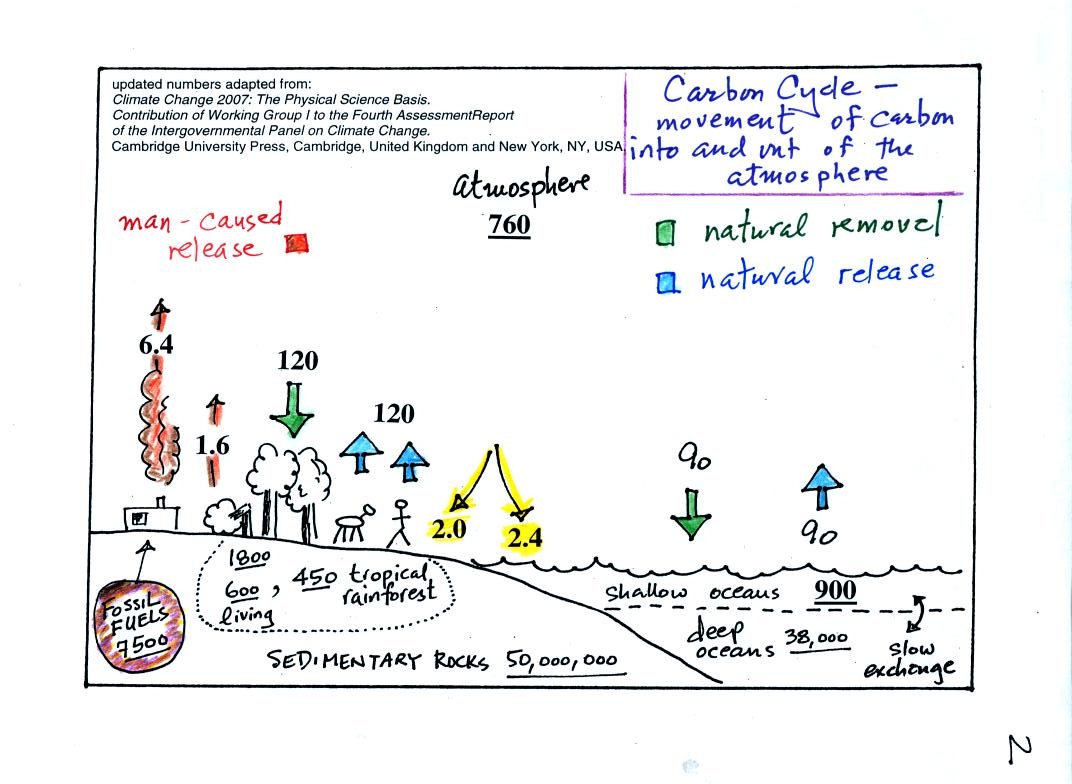
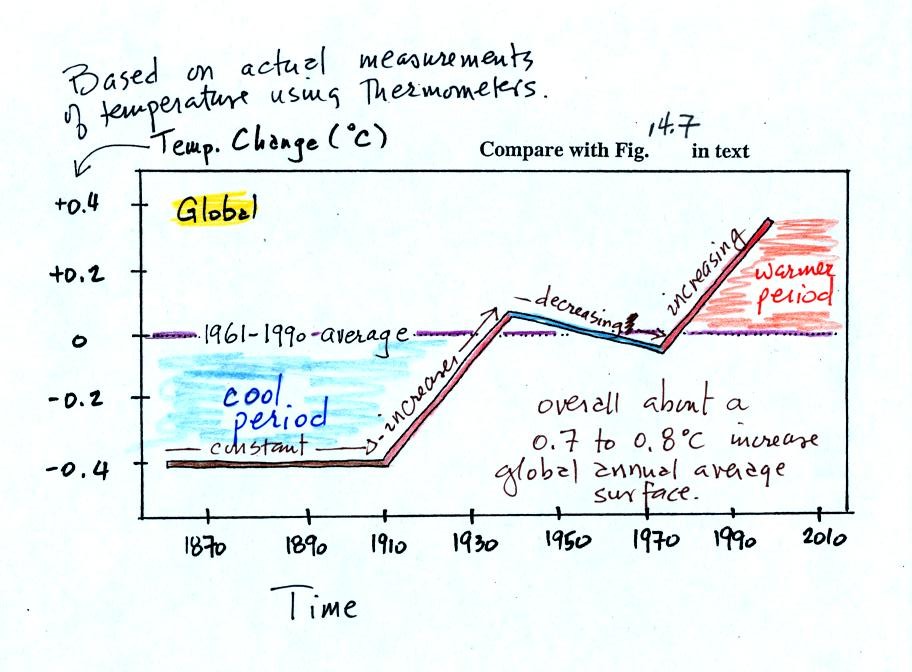
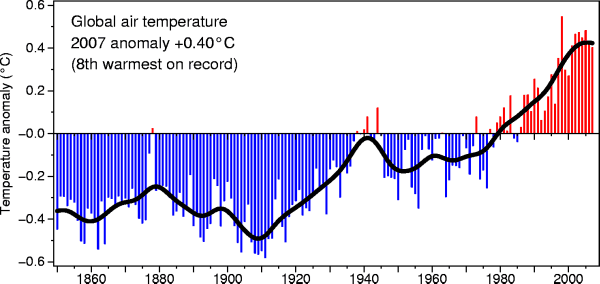
You
could walk by the house late in
the afternoon when the students would likely be outside and count
them.
That
would be a direct measurement (this would be like measuring temperature
with a thermometer). There could still be some errors in your
measurement (some students might be inside the house and might not be
counted, some of
the people outside might not live at the house).
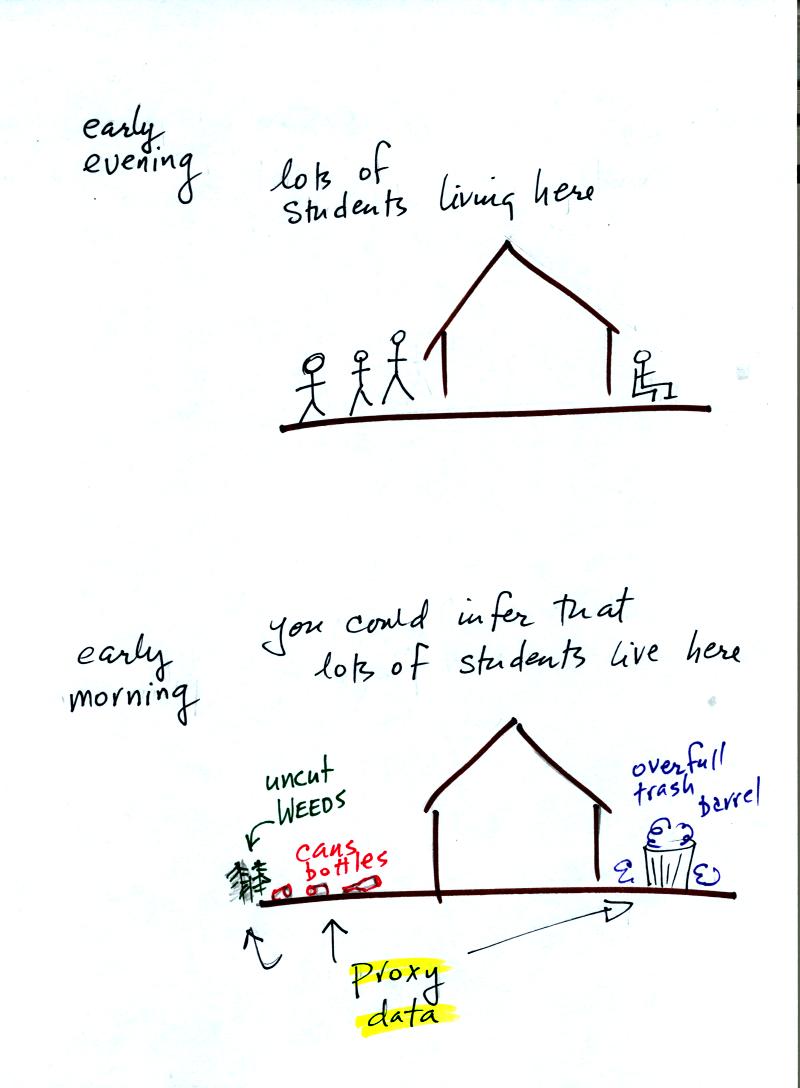
If you were to walk by early in the
morning it is likely that the
students would be inside sleeping (or in 8 am section of NATS
101). In that case you might
look for other clues (such as the number of empty bottles in the yard)
that might give you an idea of how many students
lived in that house. You would use these proxy data to come up
with an estimate of the number of students inside the house.
In the case of temperature scientists look
at a variety of
things.'
They could look at tree rings.
The width
of each
yearly ring depends on the depends on the temperature and
precipitation at the time the ring formed.
They analyze
coral.
Coral is
made up of calcium carbonate, a molecule that
contains oxygen. The relative amounts of the oxygen-16 and
oxygen-18
isotopes depends
on the temperature that existed at the time the coral grew.
Scientists can analyze lake bed and ocean
sediments.
The types
of plant and animal fossils that they find depend on
the water temperature at the time.
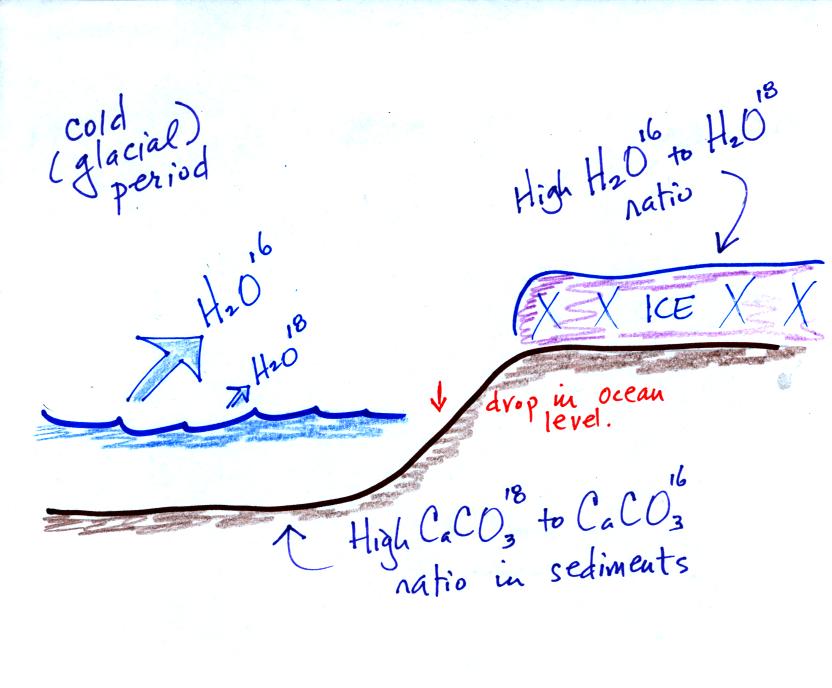
They can even use the ice
cores.
The ice, H2O,
contains
oxygen
and
the relative
amounts of
various oxygen isotopes depends on the temperature at the time the ice
fell from the sky as snow.
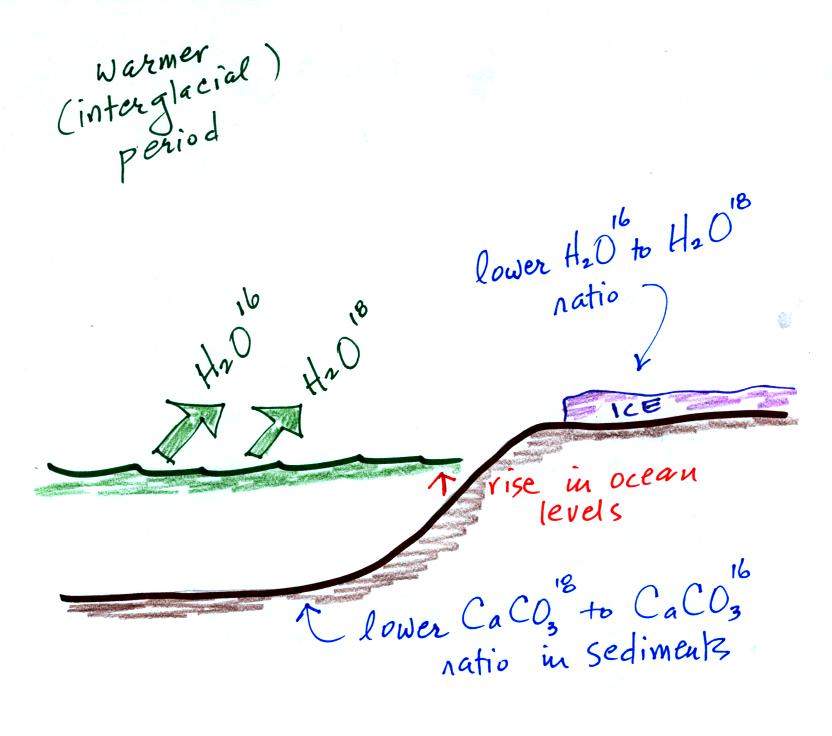
Here's an idea of how oxygen isotope data
can be used to determine past
temperature.