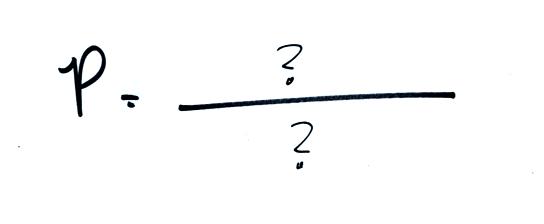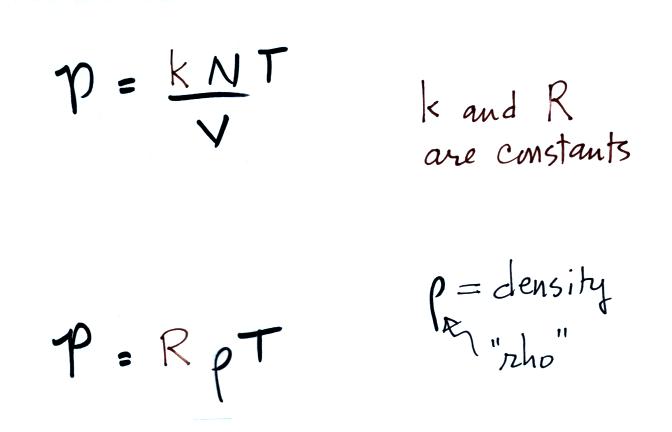If you replace the stack of bricks
with a people pyramid you
can understand that air pressure pushes upward as well as downward (it
also
pushes sideways)
Because air is compressible, now we'll use a pile of mattresses
(clean ones, not the disgusting
things you see at the curb in front of peoples homes) to help
understand that air density decreases with
increasing altitude.
Here's a more clearly drawn version of the figure were used
in class.
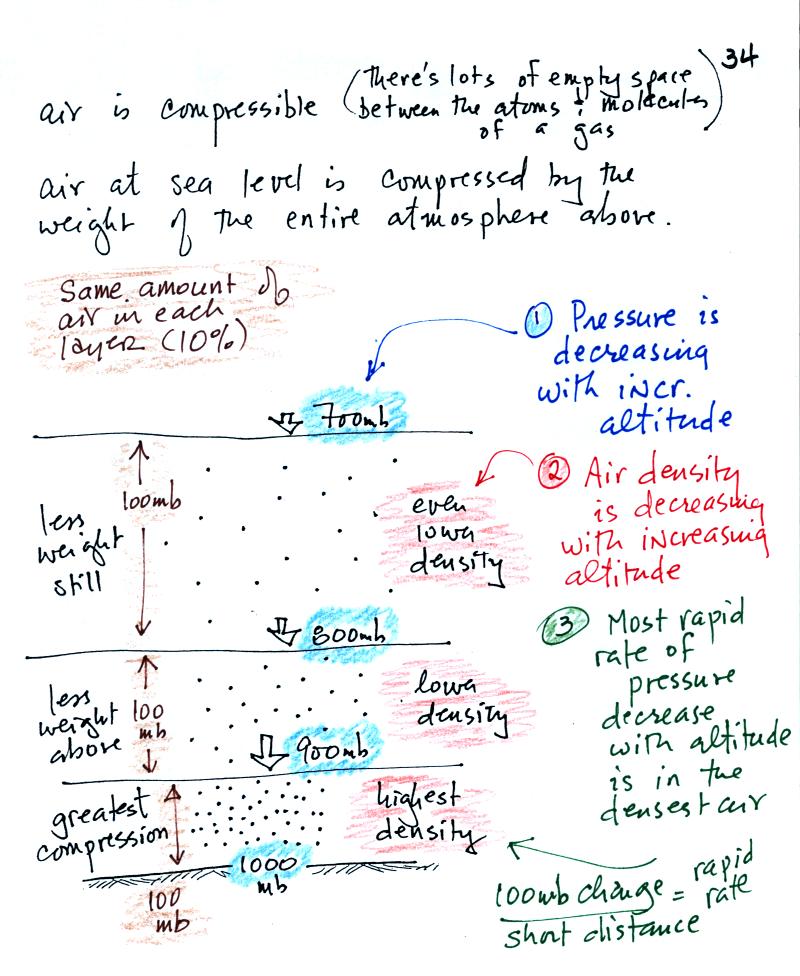
There's a lot of information in
this figure. It is worth
spending a minute or two looking at it and thinking about it.
1. You can first notice and remember that pressure
decreases
with increasing altitude. 1000 mb at the bottom decreases to 700
mb at the top of the picture.
Each layer of air contain the same amount (mass) of air. You
can
tell because the pressure decrease as you move upward through each
layer is the same (100 mb). Each layer contains 10% of the air in
the atmosphere and has the same weight.
2. The densest air is found in the bottom
layer. That is because each layer has the same amount of air
(same mass). The bottom layer is compressed the most so it is the
thinnest layer and has
the
smallest volume. Mass/( small volume)
gives a high density. The top layer has the same amount of air
but about twice the volume. It therefore has a lower density.
3. You again notice something that we covered earlier: the most
rapid
rate of pressure decrease with increasing altitude is in the densest
air in the bottom air layer. It takes almost twice the distance
for pressure to decrease from 800 mb to 700 mb in the top most layer
where the air density is lower.
Pressure decreases with increasing altitude, so does
density. What about temperature? Our
experience tells us that temperature decreases with increasing
altitude. This is what people thought was true throughout the
atmosphere up until about 1900. Then they start sending balloons
up to high altitudes in the atmosphere to measure temperature.
They were surprised
when they found that temperature stopped decreasing at an altitude of
about 10 km. Temperature actually began to increase at altitudes
above 20 km.
We had a quick look at how air temperature changes
with
altitude. The figure drawn in
class has been split into two parts and redrawn for
improved
clarity (actually this is a figure from the Fall 2008 semester).
The atmosphere can be split
into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. There are
additional layers (the mesosphere and the thermosphere) above 50 km but
we won't worry about them.
1. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature usually decreases
with
increasing altitude. [the troposphere is usually a little higher
in the tropics and lower in the cold air at polar latitudes]
The troposphere contains most of the water vapor
in the atmosphere (the water vapor comes from evaporation of ocean
water) and is
where most of the clouds and weather occurs. The
troposphere can be stable or unstable (tropo means to turn over and
refers to the fact that air can move up and down in the
troposphere).
2a. The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are occurring. When the thunderstorm reaches the
top of the troposphere, it runs into the bottom edge of the
stratosphere which is a very stable layer. The
air can't continue to rise into the stratosphere so the cloud
flattens out and forms an anvil (anvil is the name given to the flat
top of the thunderstorm). The
flat anvil top is something
that you can go outside and see and often marks the top of the
troposphere.
2b. The summit of Mt. Everest is a little over 29,000
ft. tall and is
close to the top of the troposphere.
2c. Cruising altitude in a passenger jet is usually between
30,000 and 40,000, near or just above the top of the troposphere, and
at the bottom of the stratosphere.
3. Temperature remains constant between 10 and 20 km
and then
increases with increasing altitude between 20 and 50 km. These
two sections form the stratosphere. The stratosphere is a
very stable air layer. Increasing temperature with increasing
altitude is called an
inversion. This is what makes the stratosphere so stable.
4. A kilometer is one
thousand meters. Since 1 meter is about 3 feet, 10 km is about
30,000 feet. There are 5280 feet in a mile so this is about 6
miles (about
is usually close enough in this class).
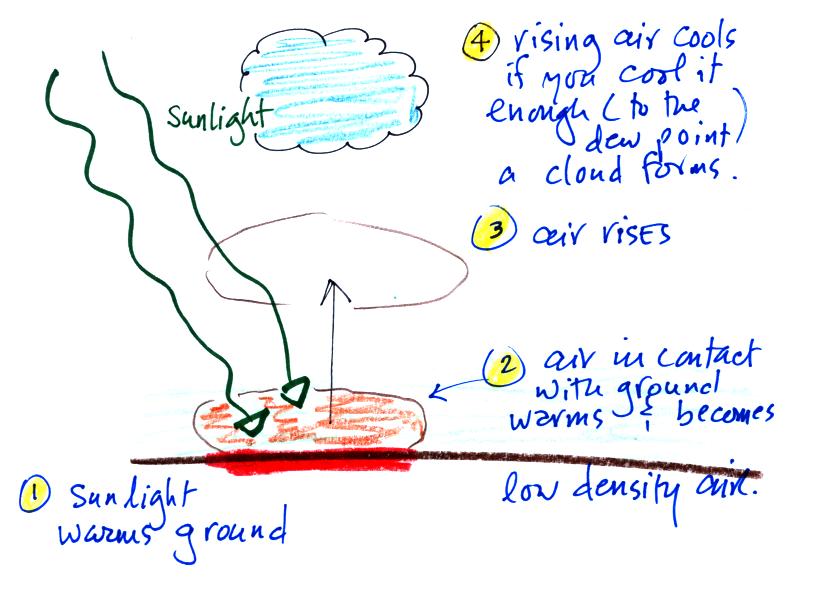
5. Sunlight is a mixture of ultraviolet (7%),
visible (44%), and
infrared light (49%). We can see the visible light.
5a. On average (over the globe and over the
course of a year) about 50% of the sunlight
arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground (20% is absorbed by gases in the air, 30% is reflected back into
space). This warms the ground. The air in contact with the
ground is warmer than air just above. As you get further and
further from the warm ground,
the
air
is
colder
and
colder.
This
explains
why air temperature decreases with increasing altitude in the
troposphere.
5b. How do you explain increasing temperature with
increasing
altitude in the stratosphere.
The ozone layer is found in the stratosphere
(peak concentrations are found near 25 km altitude). Absorption
of
ultraviolet light by ozone warms the air in the stratosphere and
explains why the air can warm. The air in the stratosphere is
much less dense (thinner) than in the troposphere. So even though
there is not very much UV light in sunlight, it doesn't
take as much energy to warm this thin air as it would to warm denser
air closer to the ground.
6. That's a manned
balloon;
Auguste Piccard and Paul Kipfer are
inside. They were to first men to travel into the
stratosphere. It really was quite a daring trip at the time at
the
time,
and they very
nearly didn't survive it. We'll see a short video segment
documenting their trip before the Practice Quiz on Thursday.
With the Experiment #1 reports due
next Tuesday, we need to cover the ideal gas law. This is also
the
first step in understanding why warm air rises and cold air
sinks.
Hot air balloons rise (they also
sink), so does the relatively
warm air in a thunderstorm (it's warmer than the air around
it). Conversely cold air sinks. The surface winds
caused by a thunderstorm downdraft (as shown above) can reach speeds of
100 MPH and are a serious weather hazard.
A full understanding of these rising and sinking motions is a
3-step process (the following is
from the bottom part of p. 49 in the photocopied ClassNotes)
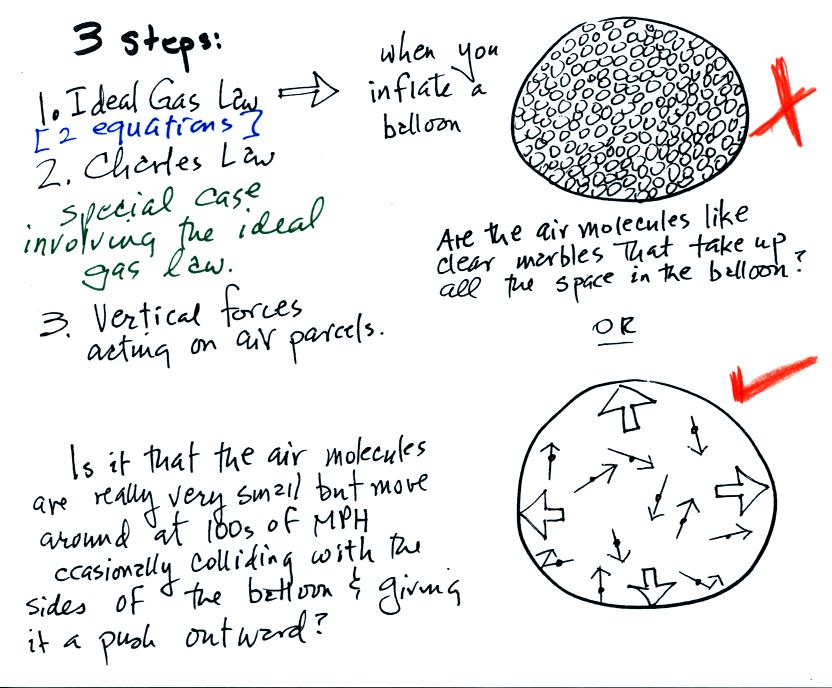
We will first learn about the ideal
gas law.
That is an equation that tells you which/how properties of the air
inside a
balloon work to determine the air's pressure. Then we will look
at Charles' Law, a special situation involving the ideal gas law (air
temperature and density change together in a way that keeps the
pressure
inside a balloon constant). Then we'll look at the
two forces that determine whether a parcel of air will rise or
sink. Only the first section on the
ideal gas law will be covered on the Practice Quiz this week.
The figure above makes an important point: the air molecules in a
balloon "filled with air" really take up very little space. A
balloon filled with air is really mostly empty space. It is the
collisions of the air molecules with the inside walls of the balloon
that keep it inflated.

This figure wasn't
shown in class. Up to this point in the semester we
have been thinking of pressure as
being determined
by the weight of the air overhead. Air pressure pushes down
against the ground at sea level with 14.7 pounds of force per square
inch. If you imagine the weight of the atmosphere pushing down on
a balloon sitting on the ground you realize that the air in the balloon
pushes back with the same force. Air everywhere in the atmosphere
pushes upwards, downwards, and sideways.
The ideal gas law
equation is another way of thinking about air pressure, sort of a
microscopic scale version. We ignore
the atmosphere and concentrate on just the air inside the
balloon. We are going to "derive" an equation. Pressure (P)
will be on the left hand side. Properties of the air inside the
balloon will be found on the right side of the equation.
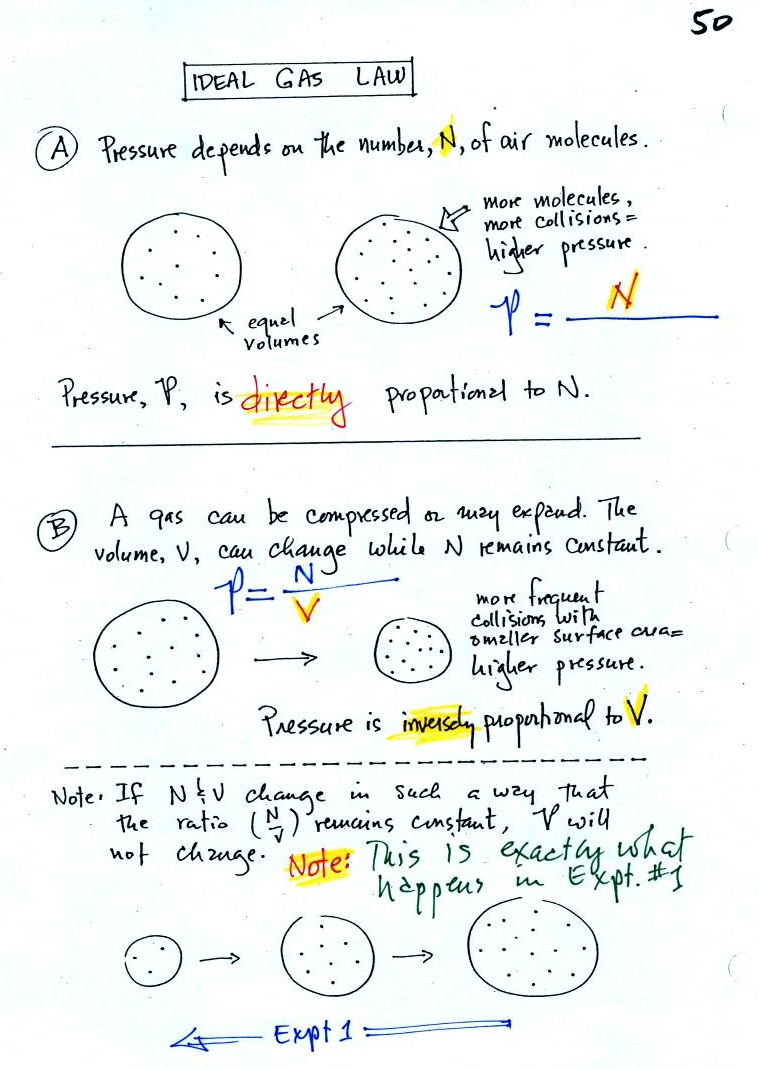
In A
the pressure produced by
the air
molecules inside a balloon will
first depend on how many air molecules are there, N. If there
weren't any air molecules at all there wouldn't be any
pressure. As you add more and more add to something like a
bicycle tire, the
pressure increases. Pressure is directly proportional to N - an
increase in N causes an increase in P. If N doubles, P also
doubles (as long as the other variables in the equation don't change).
In B
air pressure inside a balloon
also
depends on the size of the
balloon. Pressure is inversely proportional to volume, V
. If V were to double, P would drop to 1/2 its original value.
Note
it
is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
Experiment #1 that some students are working on. Oxygen in a
graduated cylinder reacts with steel wool to form rust. Oxygen is
removed from the air sample which is a decrease in N. As oxygen
is removed, water rises up into the cylinder decreasing the air sample
volume. N and V both decrease in the same relative amounts and
the air sample pressure remains constant.
If you were to remove 20% of the air molecules, V would decrease
to 20% of its original value and pressure would stay constant.
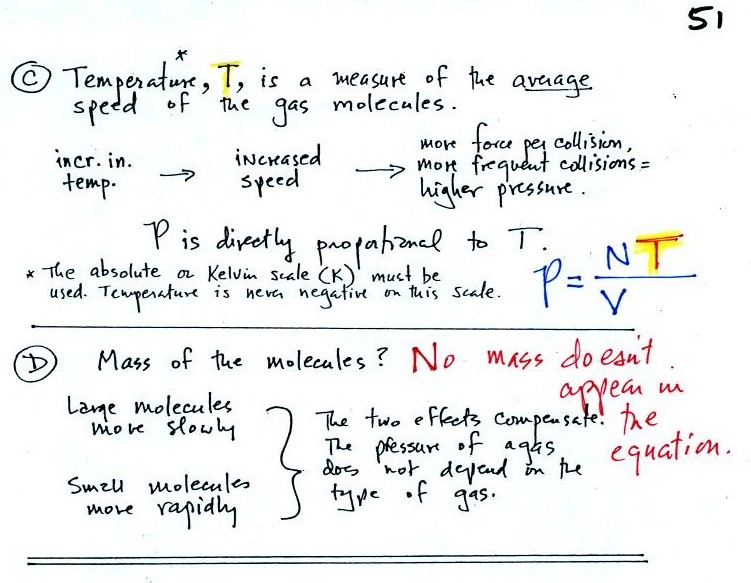
Part C: Increasing
the temperature of the gas in a balloon will cause the gas molecules to
move more quickly. They'll collide with the walls of the balloon
more frequently and rebound with greater force. Both will
increase the pressure. You shouldn't throw a can of spray paint
into a fire because the temperature will cause the pressure inside the
can to increase and the can could explode.
Surprisingly, as explained in Part
D,
the pressure
does
not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
The figure below (which replaces the bottom of p. 51 in the
photocopied
ClassNotes) shows two forms of the ideal gas law. The top
equation is the one we just derived and the bottom is a second slightly
different version. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers (which we
won't be doing).
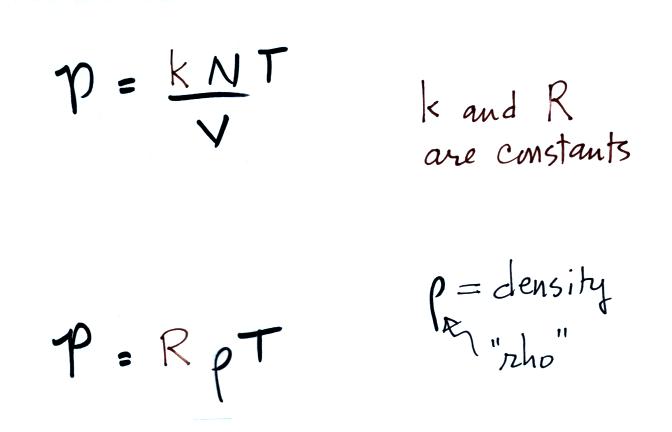
Charles' Law is a special case involving the ideal gas law.
Charles Law requires that the pressure in a volume of air remain
constant. T, V, and density can change but they must do so in a
way that keeps P constant. This is what happens in the
atmosphere. A volume of air is free to expand or shrink. It
does so to keep the pressure inside the air volume constant (the
pressure inside the volume is staying equal to the pressure of the air
outside the volume).
Read through the explanation on p.
52 in the photocopied
Classnotes. In the atmosphere a parcel (balloon) of air will
always try to keep its pressure the same as the pressure of the
surrounding air. If they aren't equal the parcel will either
expand or shrink until they are again equal.
If you warm air it will expand and density will decrease until the
pressure inside and outside the parcel are equal.
If you cool air the parcel will shrink and the density will increase
until the pressures balance.
These two associations:
(i)
warm air = low
density air
(ii) cold air = high density air
are important and will come up a
lot during the remainder of the
semester.
Click here
if you
would like a little
more detailed, more step-by-step,
explanation of Charles Law. Here's a visual summary
of Charles' Law (the
following
figure wasn't shown in class)
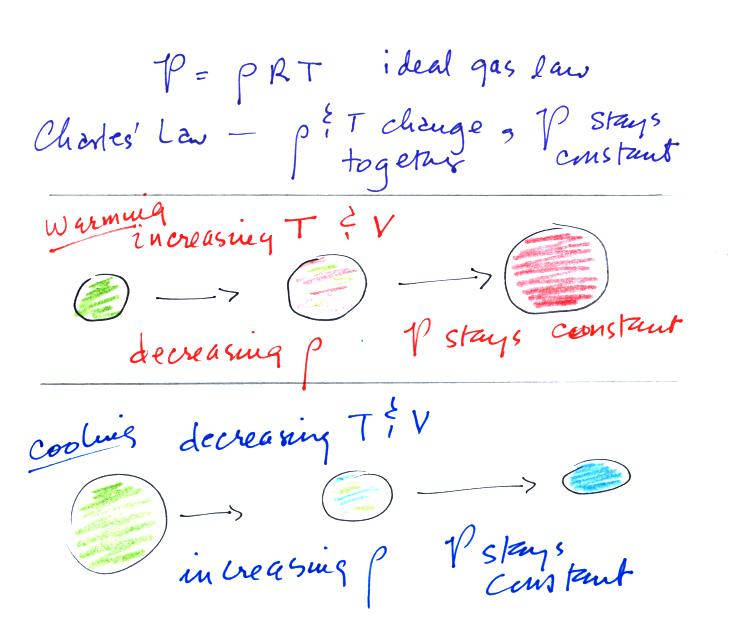
If you warm a parcel of air the
volume will increase and the density will decrease. Pressure
inside the parcel remains constant. If you cool the parcel of air
it's volume decreases and its density increases. Pressure inside
the parcel remains constant.
Charles
Law can be demonstrated by dipping a balloon in
liquid
nitrogen. You'll find an explanation on the top of p. 54 in the
photocopied ClassNotes.

The balloon had shrunk down to
practically zero volume when
pulled from the liquid nitrogen. It was filled with cold high
density air. As
the balloon warmed the balloon expanded and the density of the air
inside
the balloon decreased. The volume and temperature kept changing
in a way that kept pressure constant. Eventually the balloon ends
up back at room temperature (unless it pops).
Now
finally on to step #3. It's found on p. 53 in the photocopied
ClassNotes.
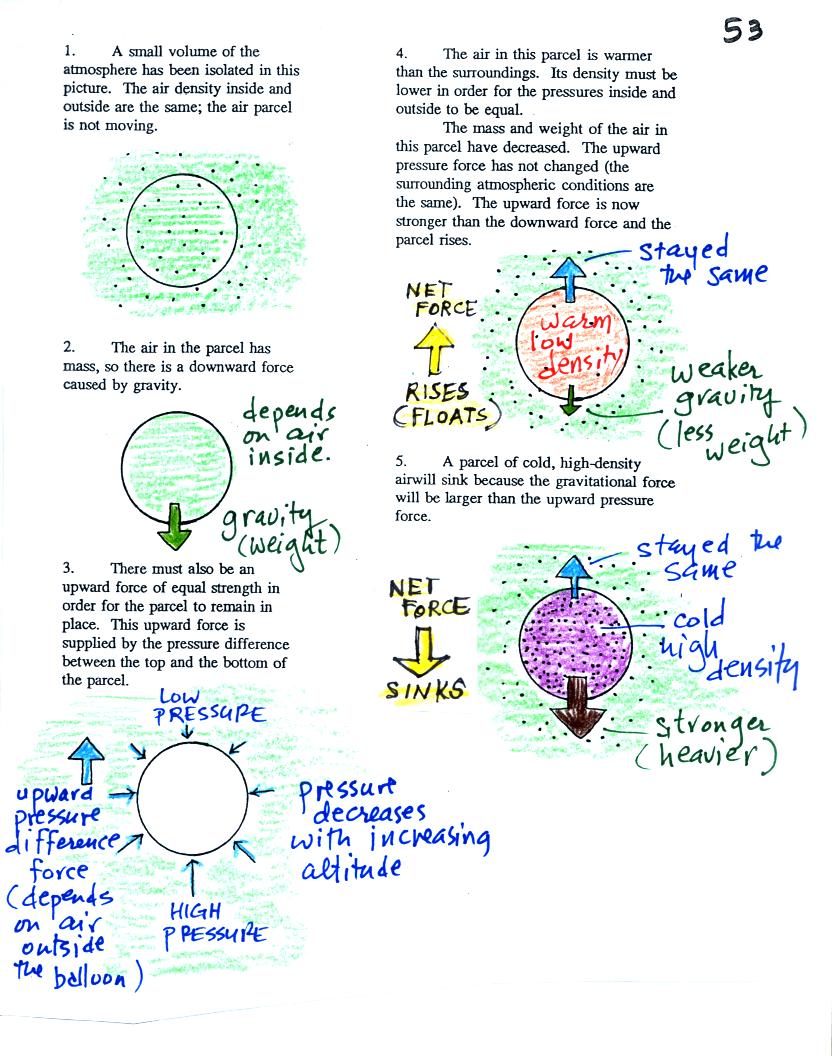
Basically it comes down to is
this - there are two forces
acting on a parcel (balloon) of air in the atmosphere:
1. Gravity pulls downward. The strength of the gravity force
depends
on the mass of the air inside
the balloon.
2. There is an upward pointing pressure difference force.
This
is
caused by the air outside
(surrounding) the balloon.
When the air inside a parcel is exactly the same as the air
outside,
the two forces are equal strength and cancel out. The parcel is
neutrally bouyant and doesn't rise or sink.
If you replace the air inside the balloon with warm low density
air, it
won't weigh as much. The gravity force is weaker. The
upward
pressure difference force doesn't change (because it is determined by
the air outside the balloon which hasn't changed) and ends up stronger
than the
gravity force. The balloon will rise.
Conversely if the air inside is cold high density air, it weighs
more. Gravity is stronger than the upward pressure difference
force and the balloon sinks.
We used
balloons filled with helium instead of air (see bottom of p. 54 in
the photocopied Class
Notes). Helium is less dense than air even when the
helium has the same temperature as the surrounding air. A
helium-filled balloon doesn't need to warmed up in order to rise.
We dunked the helium-filled balloon
in some liquid nitrogen to cool
it
and to cause the density of the helium to increase. When
removed
from the liquid nitrogen the balloon didn't rise, the gas inside was
denser than the surrounding air (the purple and blue balloons in the
figure above). As the balloon warms and expands
its density decreases. The balloon at some point has the same
density as the air around it (green above) and is neutrally
bouyant. Eventually the balloon becomes less dense that the
surrounding air (yellow) and floats up to the ceiling.
Something like this happens in the
atmosphere.