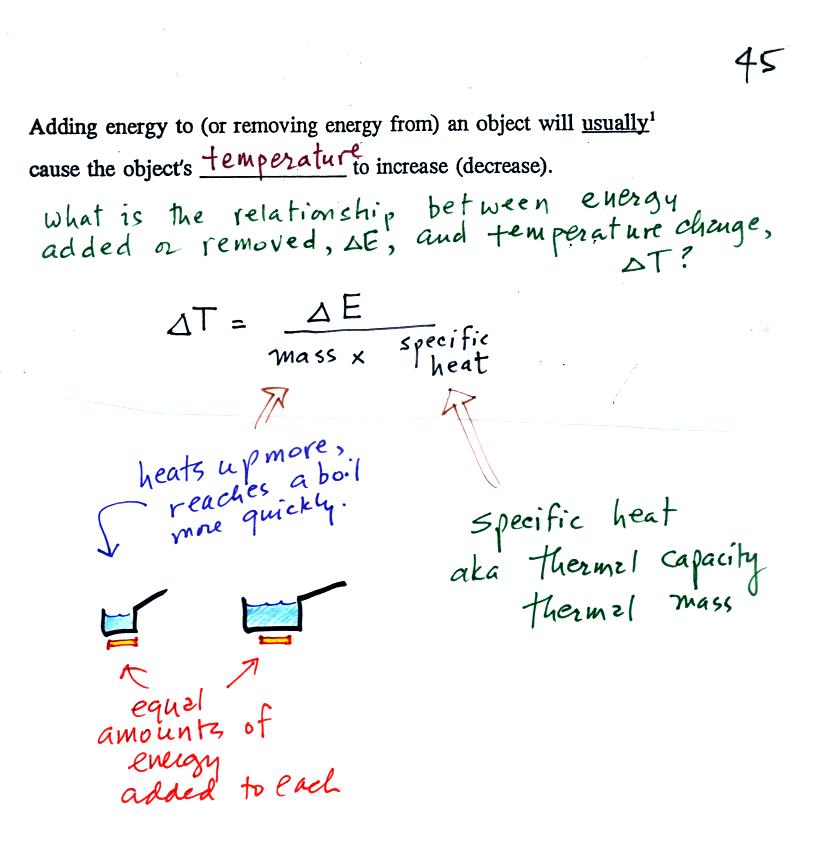
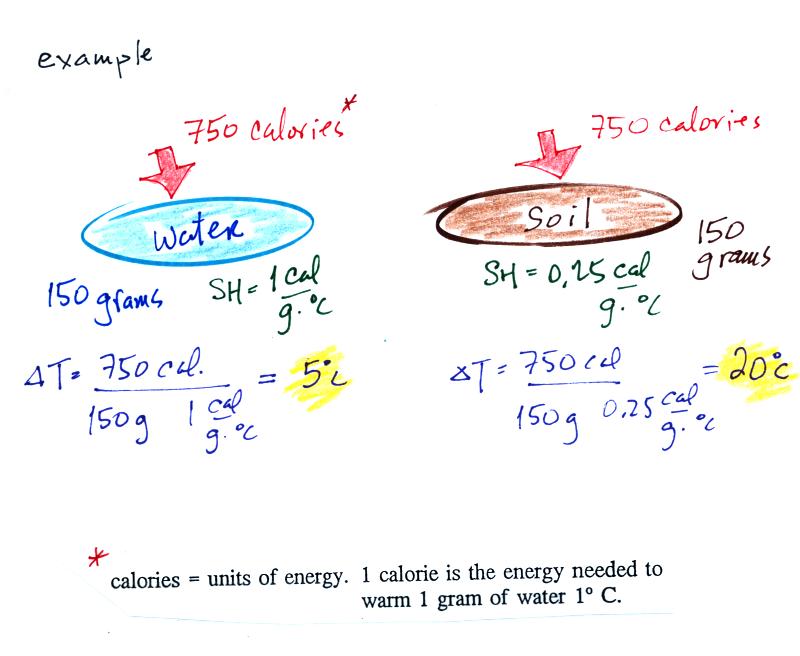
If you add energy to or remove
energy from an object, the
object
will usually change temperature. You can calculate the
temperature change if you know the object's mass and its specific
heat. That's the equation we used in the example calculation
earlier.

(a)
Some room temperature water poured into a styrofoam cup weighed
120.1
g. The cup itself weighed 3.3 g, so we had 116.8 g of water.
(b)
The water's temperature was 22.0 C (room temperature).
(c)
36.0 g of liquid nitrogen was poured into the cup of water.
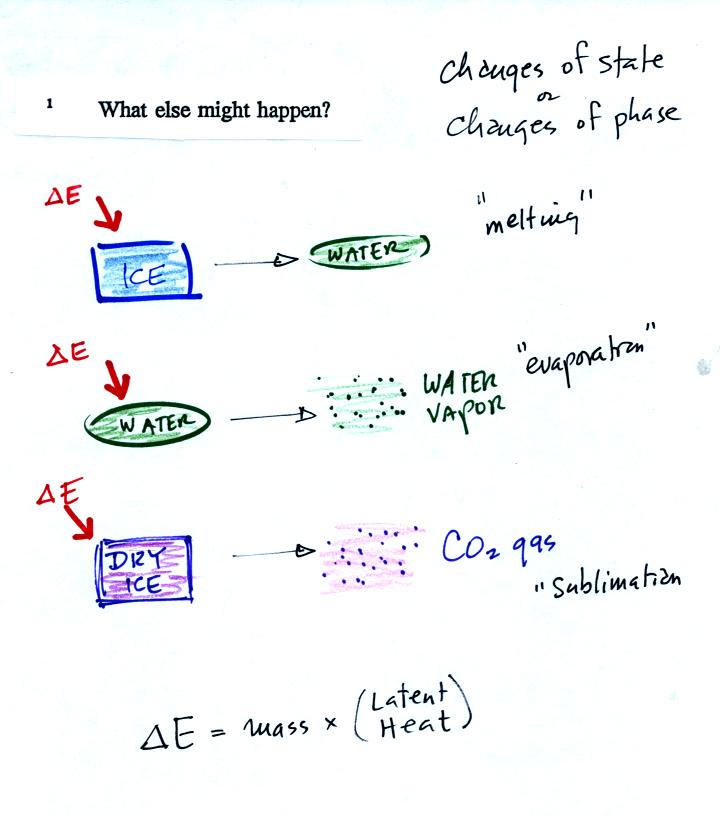
It takes energy to turn liquid nitrogen into nitrogen gas.
The needed energy came from the water. This flow of energy is
shown in the middle figure above. We assumed that because the
experiment is performed in a styrofoam cup that there is no energy
flowing between the water in the cup and the surounding air.
(d)
After the liquid nitrogen had evaporated we remeasured the water's
temperature. It had dropped to 5.1 C. That is a
temperature drop of 22.0 C - 5.1 C = 16.9 C
Because we knew how
much water we started with, its temperature drop, and water's specific
heat we can calculate how much
energy was taken from the water.
116.8 g. x 16.9 C x 1cal/g C = 1973.9 calories
We then divide that number by the
amount of liquid nitrogen that was
evaporated.
1973.9 cal / 36.0 g = 54.8 cal/g
A
trustworthy student in the class informed us that
the known value is 48 cal/g, so our measurement
was pretty darn close.
Here's a little more information
that we would have covered if it hadn't been for the fire alarm
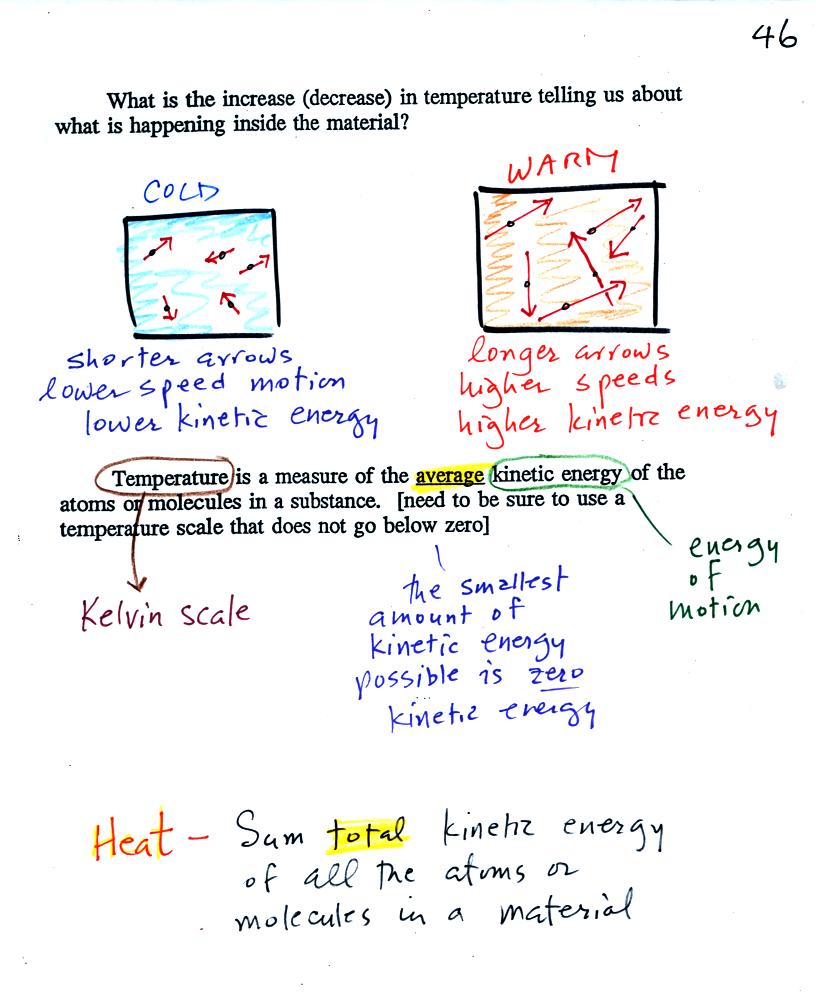
When you
add energy to an object and the object warms, what exactly is
happening inside the object?
The figure above is on p. 46 in the
photocopied Class
Notes. Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material. The atoms or molecules in a cold
material will be moving more slowly than the atoms or molecules in a
warmer object.
You need to be careful what temperature scale you use when
using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
The next figure might make the distinction between temperature (average
kinetic energy) and heat (total kinetic energy) clearer.
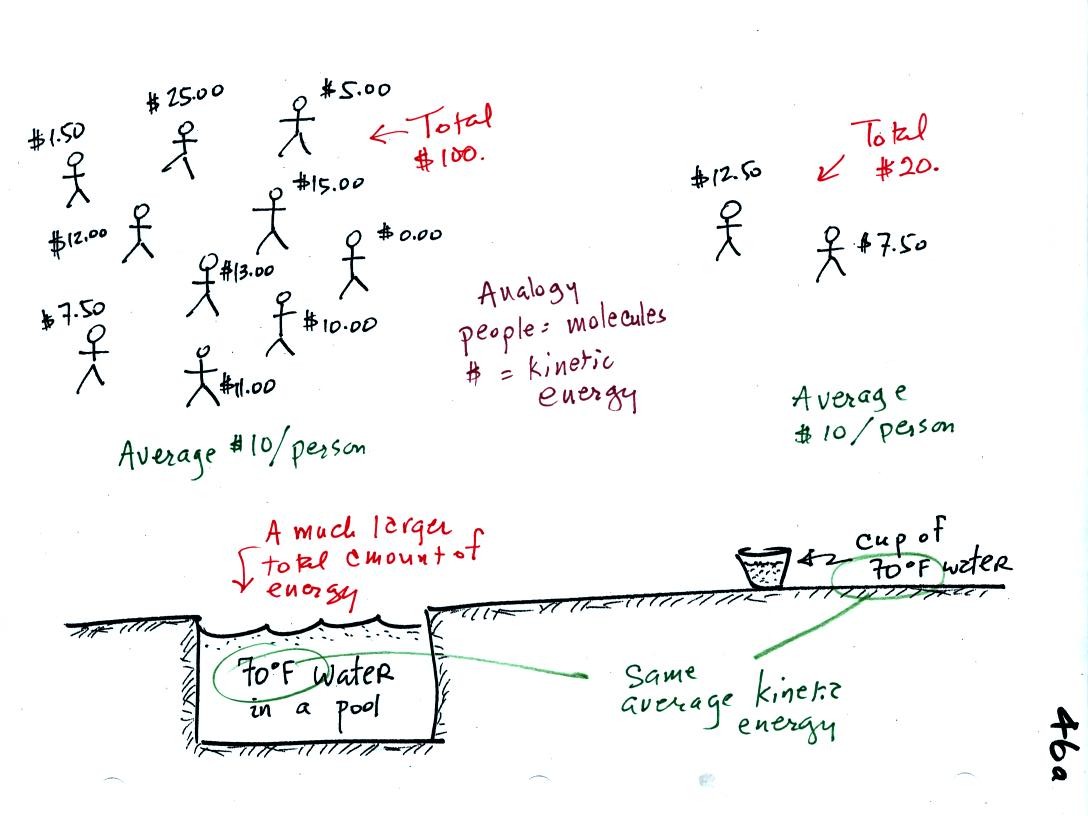
A cup of water and a pool of water
both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people shown have the same
average
amount
of money per person (that's analogous to temperature). The $100
held by the larger group at the
left is
greater than the $20 total possessed by the smaller group of people on
the right (total amount of money is analogous to heat).
Speaking
of temperature scales.
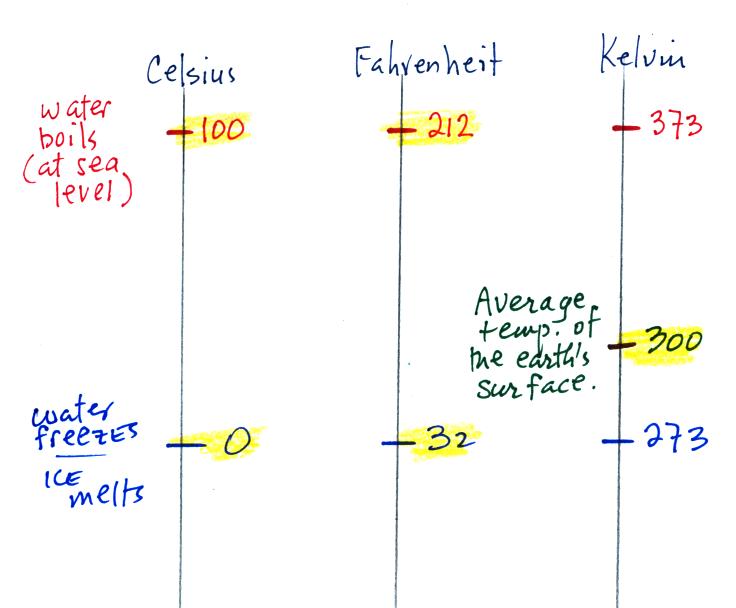
You should remember the
temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and perhaps the Kelvin
scales. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth.
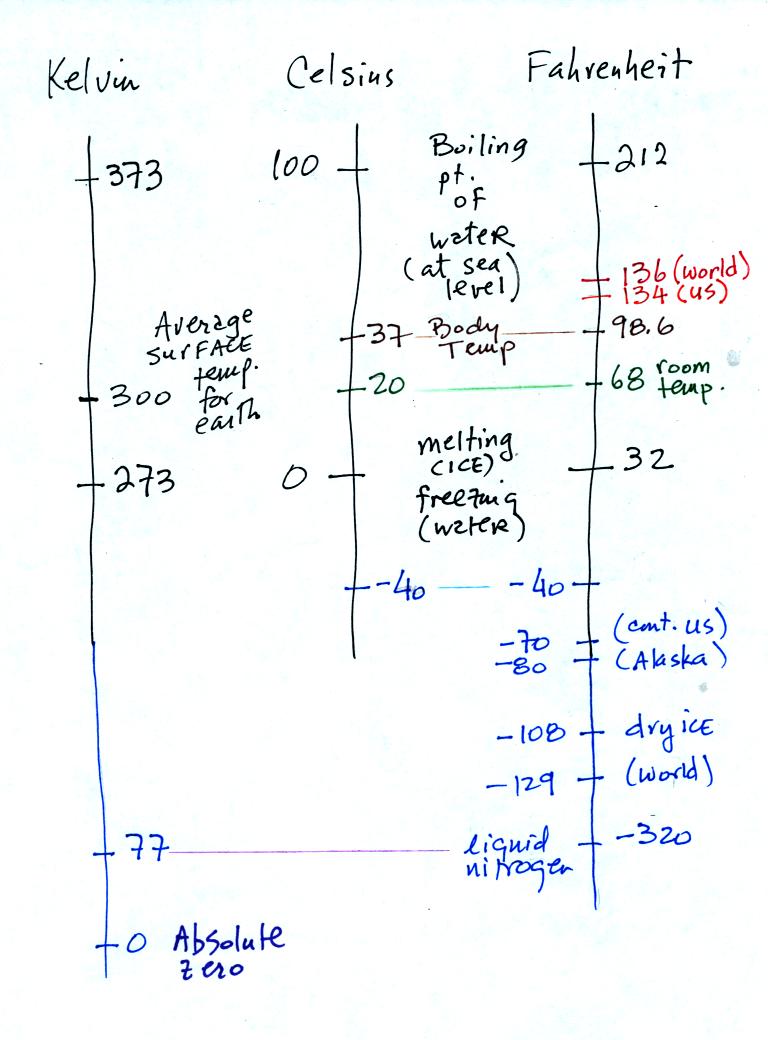
You certainly don't need to try to
remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean. Liquid
nitrogen is cold but it is still quite a bit warmer than absolute zero.