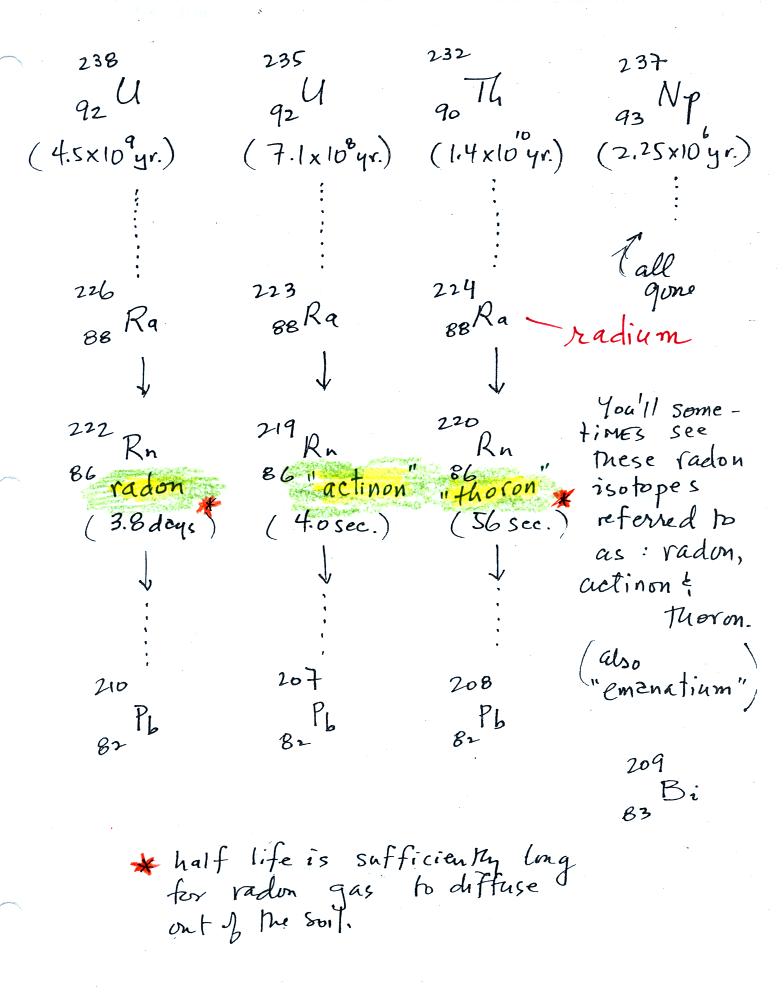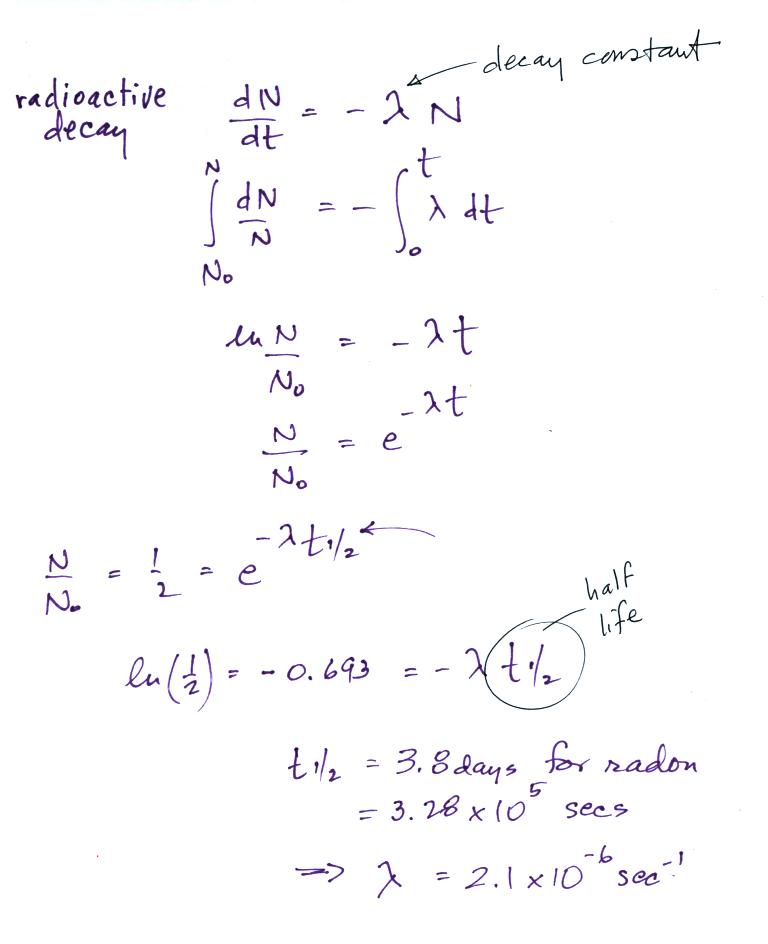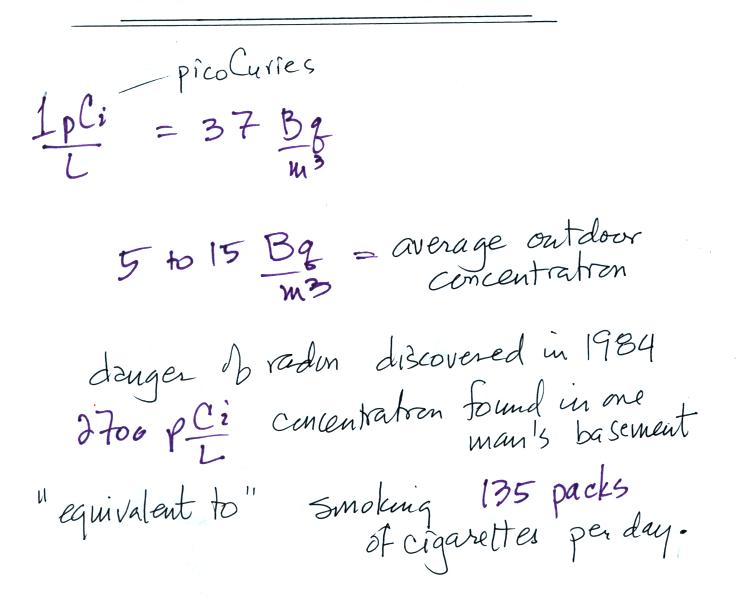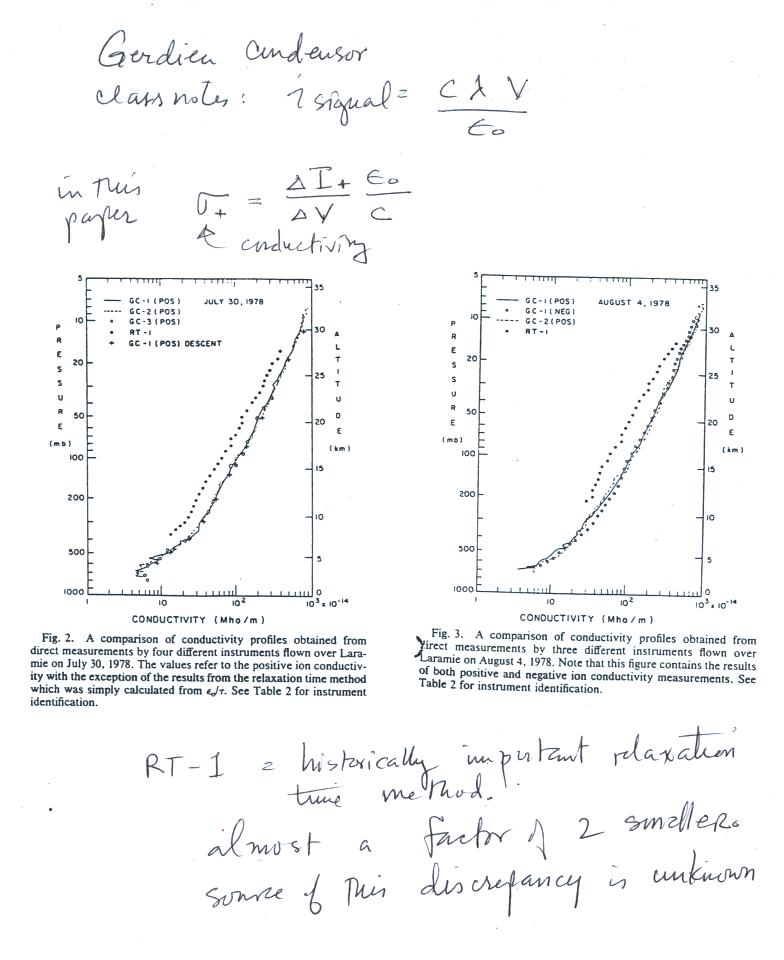




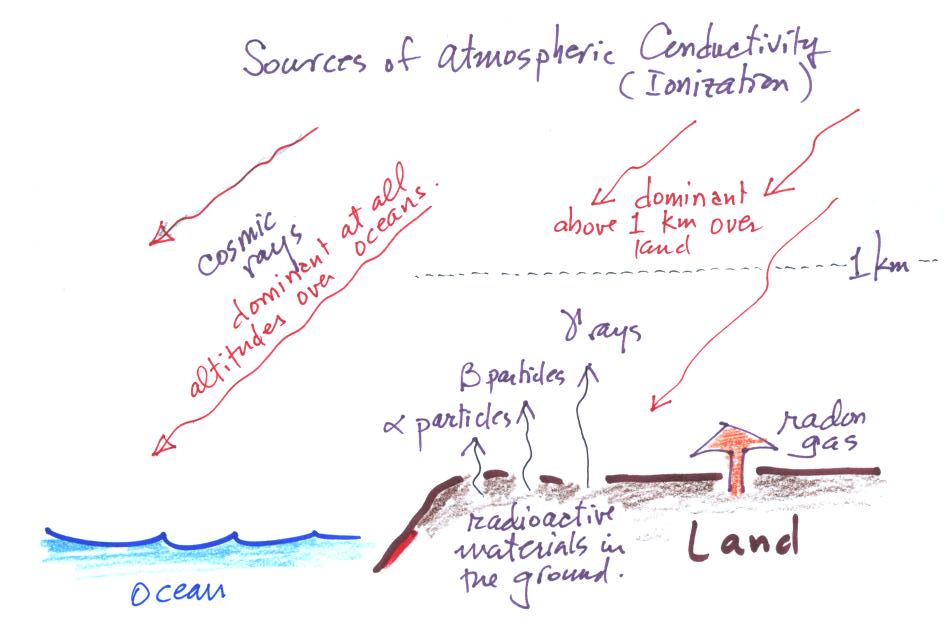
| emission type |
range of travel |
ionization rate [ ip/(cm3
sec) ] |
| alpha particles |
only a few cm above the ground |
not well
known |
| beta particles |
a few meters above the ground |
0.1 to 10 |
| gamma rays |
100s of meters above the ground |
1 to 6 |
| radon |
depends on atmospheric conditions |
1 to 20 at
1-2 m above ground |
| cosmic rays |
1
to
2
ip/(cm3
sec)
near
the ground |
|