
The right hand side of the equation
contains an ionization rate (production term) and a recombination (loss
term). Today we will add two additional small ion loss
terms. A small ion can attach to an uncharged particle, creating
a charged particle, a so-called "large ion". Or a small ion of
one polarity can attach to a charged particle of the opposite polarity
creating an unchared particle (if the small ion and particle have equal
quantities of charge). These two new terms are shown at the top
of the figure below.
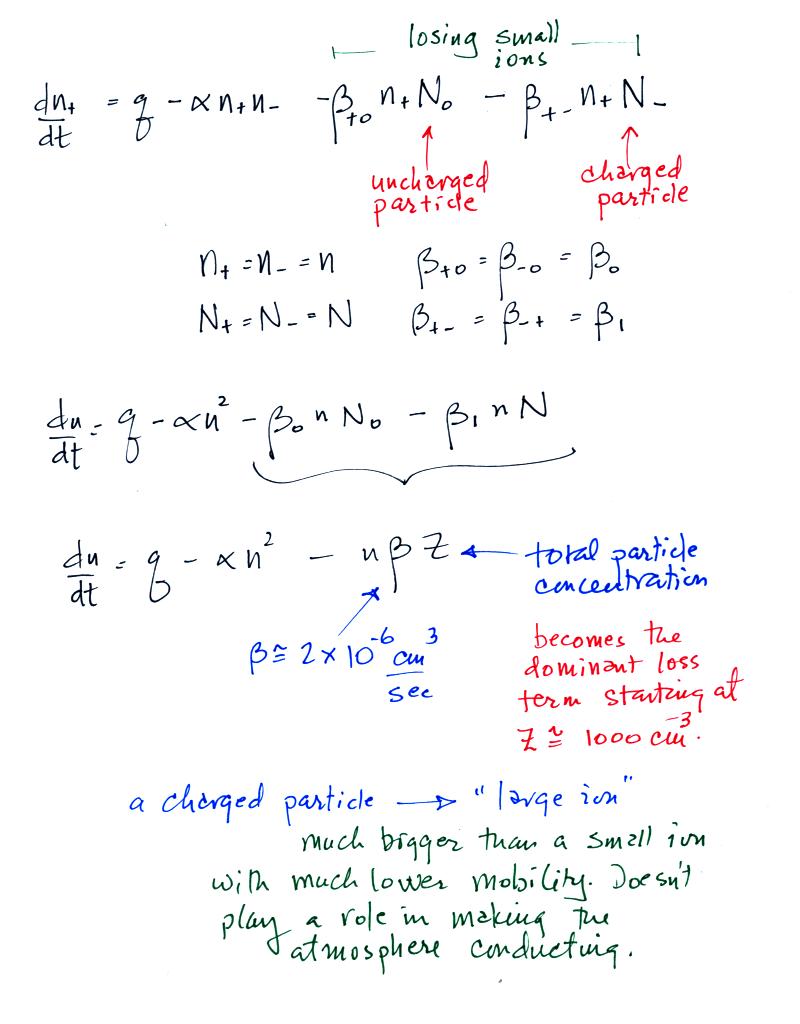

The original small ion - particle
balance equation is often simplified considerably to include just a
total particle concentration term, Z, rather than keeping track
of the concentrations of charged and uncharged particles.
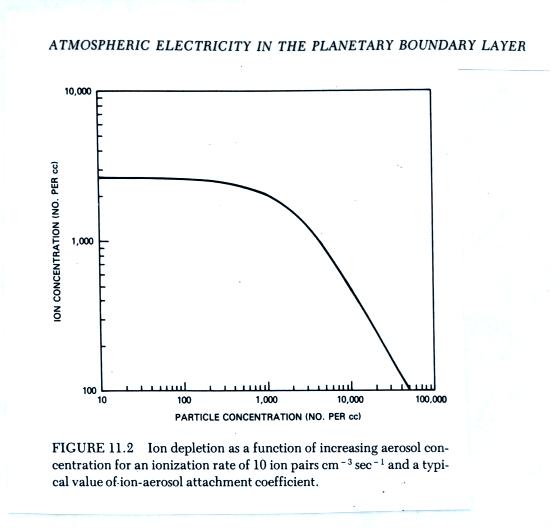
The figure below (from The Earth's Electrical Environment (the "yellow book") reference) illustrates how ion-particle attachment begins to significantly reduce small ion concentrations beginning at particles concentrations of about 1000 cm-3
In some past editions of this course we have spent close to a full class period looking at how you might derive expressions for the ion-particle attachment coefficients. I decided not to do that this semester. Though I will add some notes that you can look at if you are interested (it might be a while before they appear). The first part of these supplementary notes deals with the attachment to uncharged particles, the second part considers attachment to charged particles.
Update. It's July 14 (Bastille Day) and I've finally got those notes done. You can find them here.
We did spend some time considering what fraction of particles are uncharged and charged.

The figure below (from The Earth's Electrical Environment (the "yellow book") reference) illustrates how ion-particle attachment begins to significantly reduce small ion concentrations beginning at particles concentrations of about 1000 cm-3

In some past editions of this course we have spent close to a full class period looking at how you might derive expressions for the ion-particle attachment coefficients. I decided not to do that this semester. Though I will add some notes that you can look at if you are interested (it might be a while before they appear). The first part of these supplementary notes deals with the attachment to uncharged particles, the second part considers attachment to charged particles.
Update. It's July 14 (Bastille Day) and I've finally got those notes done. You can find them here.
We did spend some time considering what fraction of particles are uncharged and charged.

For large particles you would
expect to find equal numbers of positively charged, negatively charged,
and non-charged particles.

The agreement between predictions and measurements of the uncharged fraction (No / Z) is not very good for small particles.
Better agreement is obtained using Boltzmann statistics. Results are shown below, we didn't look at the details of the theory or the calculation (I may add some additional details at a later time).


The agreement between predictions and measurements of the uncharged fraction (No / Z) is not very good for small particles.
Better agreement is obtained using Boltzmann statistics. Results are shown below, we didn't look at the details of the theory or the calculation (I may add some additional details at a later time).

The majority of small particles are
uncharged. Those that are charged only have a single electronic
charge.
The uncharged fraction has been computed for the 3 particle sizes in the earlier table. Results are shown below
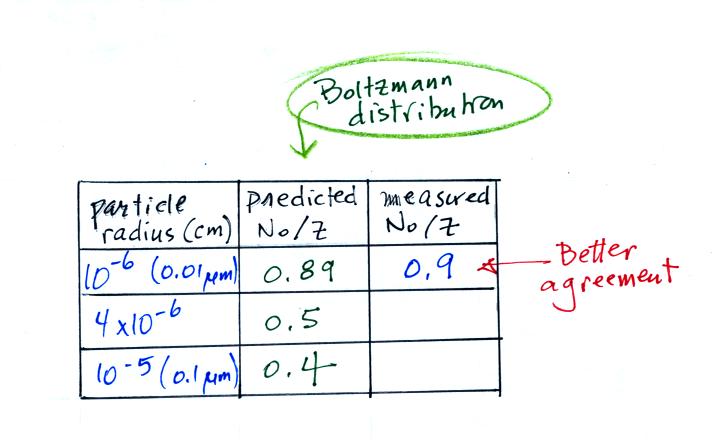
The uncharged fraction has been computed for the 3 particle sizes in the earlier table. Results are shown below

The agreement between measured and
predicted values (10-6 cm radius particles) is much better.
The next major topic we will be covering is cloud electrification.
First we need to do a little review and have a look at the structure of a cold thunderstorm cloud. Cold refers to the fact that much of the cloud is found at high enough altitude that it is at below freezing temperatures and contains ice crystals. This is the case for thunderstorm clouds even in Tucson on the hottest day in summer.
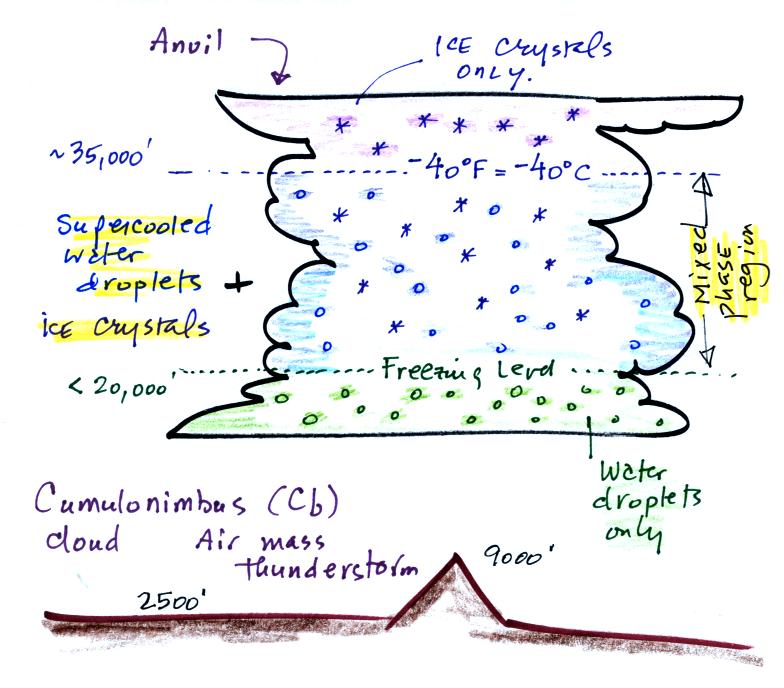
The important part of the cloud, both for precipitation
formation and electrification , is the middle mixed phase region.
There you find ice crystals and lots of supercooled water droplets
(water droplets cooled to below freezing that are unable to freeze).
We will look briefly and qualitatively at what makes it difficult for the water to freeze (unless it gets really cold, below -40 F). But first it is worth noting that the formation of water droplets does not occur as you might have imagined. You might have thought that once the RH reaches 100% that water vapor would simply condense and form little droplets.

The rate of evaporation from a small droplet is much higher than
you would find over a flat surface of water. This is called the
curvature effect. If a small droplet of pure water were to form
it would quickly evaporate. Condensation from the moist
surroundings would not be enough to overcome the high rate of
evaporation. A droplet must somehow reach a critical size (with a
corresponding drop in the rate of evaporation) before it will be in
equilibrium with its surroundings.

The next major topic we will be covering is cloud electrification.
First we need to do a little review and have a look at the structure of a cold thunderstorm cloud. Cold refers to the fact that much of the cloud is found at high enough altitude that it is at below freezing temperatures and contains ice crystals. This is the case for thunderstorm clouds even in Tucson on the hottest day in summer.

We will look briefly and qualitatively at what makes it difficult for the water to freeze (unless it gets really cold, below -40 F). But first it is worth noting that the formation of water droplets does not occur as you might have imagined. You might have thought that once the RH reaches 100% that water vapor would simply condense and form little droplets.


Particles in the air, so called
cloud condensation nuclei (CCN), make it much easier for cloud droplets
to form. Water vapor could simply condense onto a particle of
appropriate size. The water droplet would effectively start at
rather than grow to the critical size and would be in equilibrium with
its surroundings.

Some particles will dissolve when water condenses onto them.
The droplet of solution has a lower rate of evaporation than a droplet
of pure water. Once a droplet begins to grow, the solution
concentration will decrease and the effect on evaporation rate will
diminish. Note that it is possible for small "haze" droplets of
solution to form and be in equilibrium with their surroundings when the
relative humidity is less than 100%.
Ice crystals in a cloud can basically form in two ways

Water vapor can turn directly to ice or supercooled water droplets can freeze.


Ice crystals in a cloud can basically form in two ways

Water vapor can turn directly to ice or supercooled water droplets can freeze.

In both cases it is easier to make
use of an ice nucleus particle. The problem is that there aren't
many materials that can act as an ice nucleus.
Silver iodide is used in cloud seeding. Kaolinite is a clay
material (that was used at one time in Kaopectate for the treatment of
diarrhea, bismuch subsalicilate is now used). Certain bacteria
also are effective ice nuclei!? Bacteria are added to water in
snow making operations at ski resorts to ensure that the water freezes
when sprayed onto the slopes.

Ice crystals evaporate (actually they sublimate) at a slower rate than water droplets. Because the surrounding air is moist enough to keep the water droplets in equilibrium (3 arrows of condensation balancing 3 arrows of evaporation in the figure above), and because water vapor will condense onto the ice crystals at the same rate, the ice crystals will grow and become snow crystals (just bigger ice crystals).

It used to be (and maybe still is) that people would make replicas
of snow crystals by allowing them to fall onto a microscope slide
coated with formvar (a plastic resin material of some dissolved in
acetone or something like that). The crystal would melt and
evaporate but would leave behind an impression in the formvar which
would itself evaporate and harden. You could then examine or
photograph the crystal replica under a microscope. Snow crystals
come in lots of different shapes (called "habits", a plate is sketched
above) depending on the amount of moisture in the cloud and the
temperature. Have a look at
photomicrographs of some snow crystals at www.snowcrystals.com.
A couple of more things you need to be familiar with before we start talking about electrification processes.
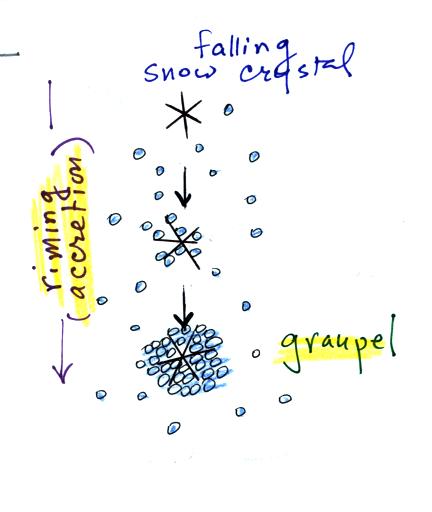
The snow crystal in the picture above is falling and colliding
with supercooled water droplets. The droplets stick and
freeze. This process is called riming or accretion. If this
goes on long enough the snow crystal can get completely covered with
frozen droplets. The resulting particle is called graupel, soft
hail, or snow pellets. Graupel particles can grow up to maybe 1/4
inch across. They have a frosty white appearance and resemble a
miniature snow ball.

Graupel is really not hail. Hail usually starts with a
graupel core and then has alternating layers of clear ice and rime ice
(the frosty white ice that makes up graupel). In Tucson hail
usually has just a graupel core and a single layer of clear ice.
The appearance is quite distinctive and clearly different from
graupel. In the big severe thunderstorms in the Central Plains
the hailstones can have many layers of rime ice and clear ice.
This is the last picture of the day. It shows the normal distribution of charge in a thunderstorm. This is what a viable cloud electrification process needs to be able to explain.

Note first of all the cloud has a rough tripolar structure
consisting of a main negative charge center (1a), an upper positive
charge center (1b), and lower positive charge centers (1c). All
are found at temperatures colder than freezing. The main layer of
negative charge (1a) seems always to be found at temperatures between
-10 C and -30 C.
Screening layers are found at the top and sides of the cloud (2a and 2b in the figure). These form because of the abrupt drop in conductivity as you move from outside the cloud into the cloud.
E fields under the thunderstorm at the ground are typically 1000s of V/m (100 to 300 V/m is typically found during fair weather). Enhancement of the E field at the points of sharp objects on the ground often go into corona discharge and spray positive charge into the air near the ground. The ground under the main part of the thunderstorm is also positively charged.

Ice crystals evaporate (actually they sublimate) at a slower rate than water droplets. Because the surrounding air is moist enough to keep the water droplets in equilibrium (3 arrows of condensation balancing 3 arrows of evaporation in the figure above), and because water vapor will condense onto the ice crystals at the same rate, the ice crystals will grow and become snow crystals (just bigger ice crystals).

A couple of more things you need to be familiar with before we start talking about electrification processes.


This is the last picture of the day. It shows the normal distribution of charge in a thunderstorm. This is what a viable cloud electrification process needs to be able to explain.

Screening layers are found at the top and sides of the cloud (2a and 2b in the figure). These form because of the abrupt drop in conductivity as you move from outside the cloud into the cloud.
E fields under the thunderstorm at the ground are typically 1000s of V/m (100 to 300 V/m is typically found during fair weather). Enhancement of the E field at the points of sharp objects on the ground often go into corona discharge and spray positive charge into the air near the ground. The ground under the main part of the thunderstorm is also positively charged.