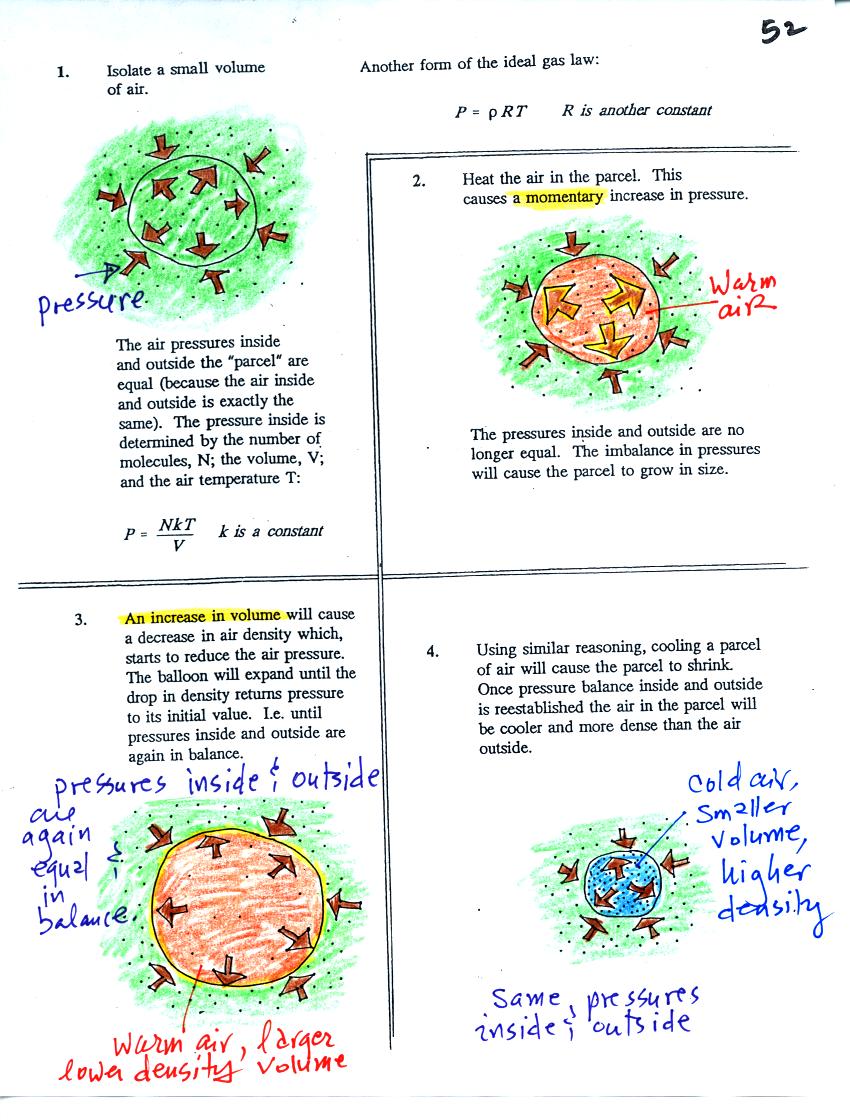
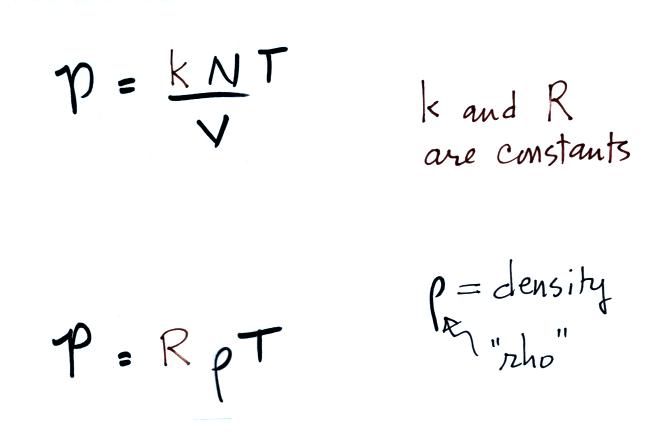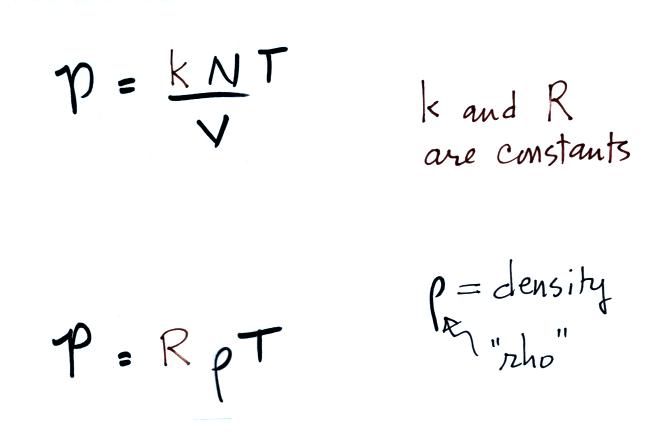If you warm a parcel of air the
volume will increase and the density will decrease. Pressure
inside the parcel remains constant. If you cool the parcel of air
it's volume decreases and its density increases. Pressure inside
the parcel remains constant.
Charles
Law can be demonstrated by dipping a balloon in
liquid
nitrogen. You'll find an explanation on the top of p. 54 in the
photocopied ClassNotes.

The balloon had shrunk down to
practically zero volume when
pulled from the liquid nitrogen. It was filled with cold high
density air. As
the balloon warmed the balloon expanded and the density of the air
inside
the balloon decreased. The volume and temperature kept changing
in a way that kept pressure constant. Eventually the balloon ends
up back at room temperature (unless it pops while warming up).
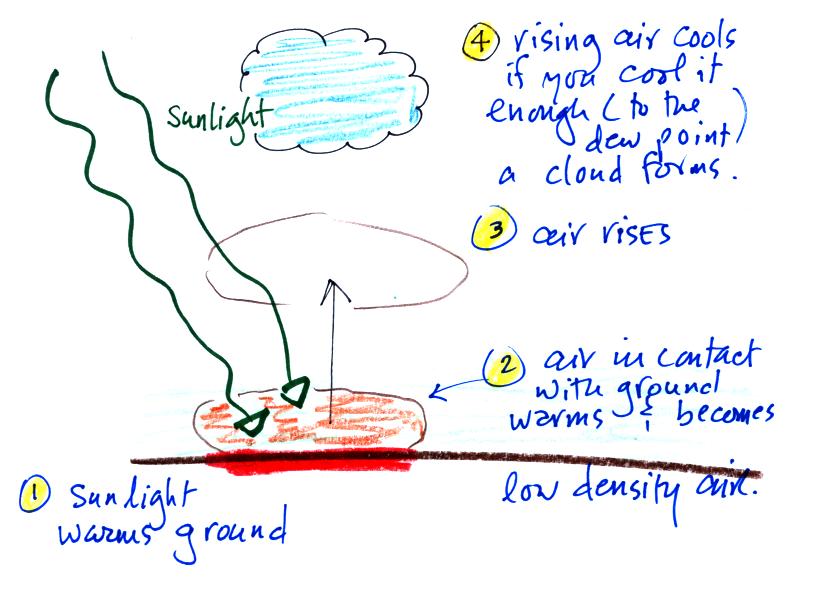
And finally the last step toward understanding why warm air rises
and cold air sinks. We'll have a look at the forces that act on
parcels of air in the atmosphere. This information is
found on p. 53
in the photocopied
ClassNotes.
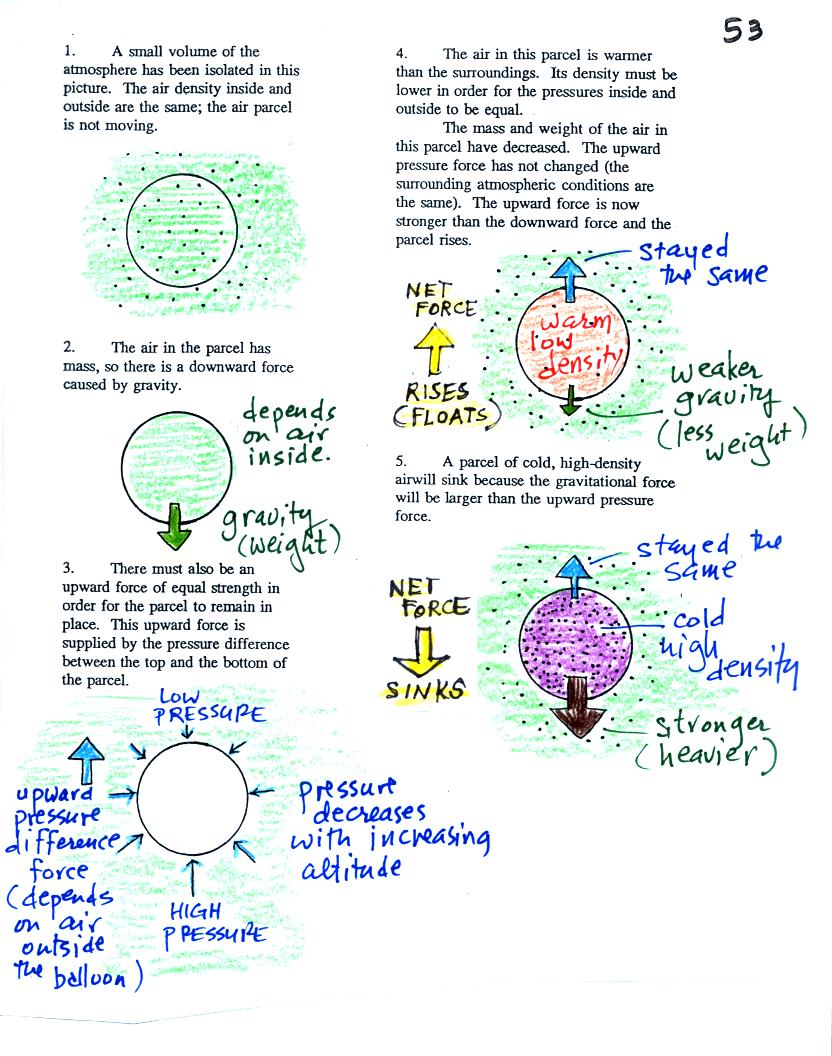
Basically it comes down to this - there are two forces
acting on a parcel* of air in the atmosphere:
1. Gravity pulls downward. The strength of the gravity force
depends
on the mass of the air inside
the parcel. This force is just the weight of the parcel
2. There is an upward pointing pressure difference force.
This
force is
caused by the air outside
(surrounding) the parcel. Pressure decreases with increasing
altitude. The pressure of the air at the bottom of a parcel
pushing upward is slightly stronger than the pressure of the air at the
top of the balloon that is pushing downward. The overall effect
is an upward pointing force.
When the air inside a parcel is exactly the same as the air
outside,
the two forces are equal in strength and cancel out. The parcel
is
neutrally bouyant and doesn't rise or sink.
If you replace the air inside the balloon with warm low density
air, it
won't weigh as much. The gravity force is weaker. The
upward
pressure difference force doesn't change (because it is determined by
the air outside the balloon which hasn't changed) and ends up stronger
than the
gravity force. The balloon will rise.
Conversely if the air inside is cold high density air, it weighs
more. Gravity is stronger than the upward pressure difference
force and the balloon sinks.
* the
word
parcel
just
means
a
small volume of air.
We did a short demonstration to show how density can
determine
whether an object or a parcel of air will rise or sink. We used
balloons filled with helium (see bottom of p. 54 in
the photocopied Class
Notes). Helium is less dense than air even when the
helium has the same temperature as the surrounding air. A
helium-filled balloon doesn't need to warmed up in order to rise.
We dunked the helium-filled balloon
in some liquid nitrogen to cool
it
and to cause the density of the helium to increase. When
removed
from the liquid nitrogen the balloon didn't rise, the gas inside was
denser than the surrounding air (the purple and blue balloons in the
figure above). As the balloon warms and expands
its density decreases. The balloon at some point has the same
density as the air around it (green above) and is neutrally
bouyant. Eventually the balloon becomes less dense that the
surrounding air (yellow) and floats up to the ceiling.
Something like this happens in the
atmosphere.
In
the last few minutes of class we learned a little bit
about the Piccard family.
Auguste Piccard
(1884-1962) together with Paul Kipfer (see p. 32 in the
photocopied ClassNotes) was the lead member of a two-man team that made
the
first trip into the stratosphere in a balloon. They did that on
May 27,
1931. We watched a short segment from a PBS program called "The
Adventurers" that documented that trip.
Jacques Piccard
(Auguste's son) was part of a
two-man team that traveled to
the deepest point in the ocean (35,800 feet) in a bathyscaph. In
the next
week or so I will show you a short segment from an earlier test of the
bathyscaph where Auguste and Jacques descended to 10,000 feet.
Finally Bertrand
Piccard (Jacques son, Auguste's
grandson) was part of the two man team that first circled the globe
nonstop in
a balloon. That occurred much more recently, March 20, 1999, I
believe. I also plan to show you some of that trip.