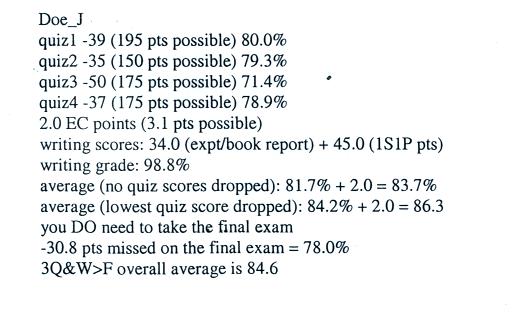
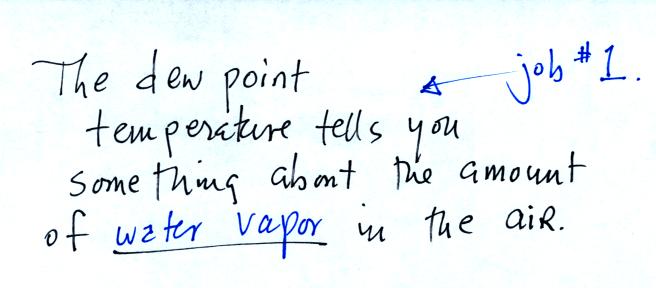The chart below gives a rough
equivalence between dew point
temperature and percentage concentration of water vapor in the air.
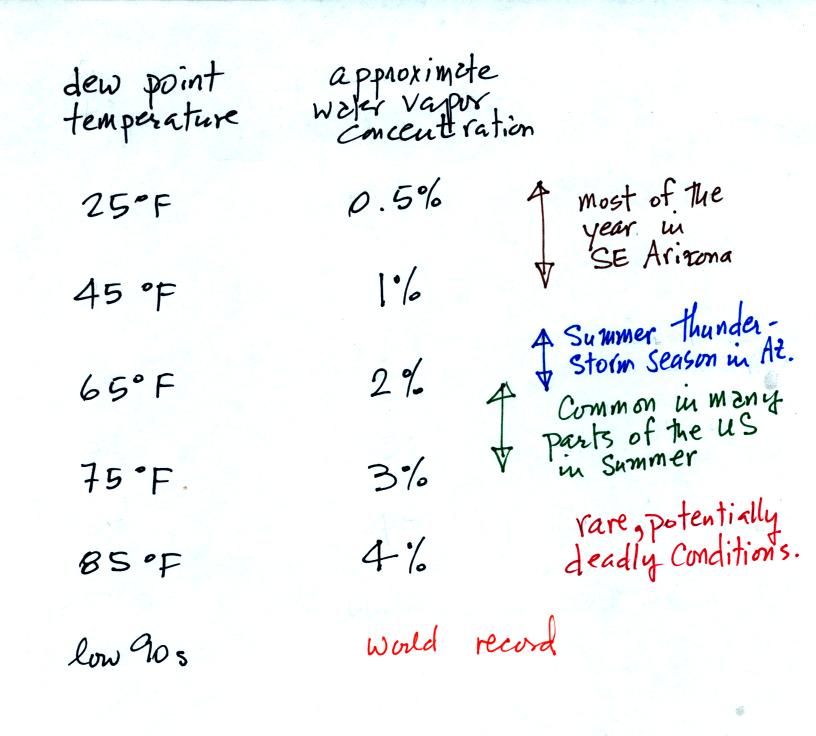
Air temperature will
always be equal to or warmer than
the dew point
temperature. Experiencing 80o dew points would be very
unpleasant (and possibly life threatening because your body might not
be able to cool itself). Click
here
to see current dew point temperatures across the U.S. Don't worry
about remembering all these numbers. Just remember that the
higher the dew point temperature the more water vapor is in the air and
vice versa. As you saw in class, I have trouble remembering these
numbers. If I can't remember them I can't really expect you to.
The second job of the dew point temperature is
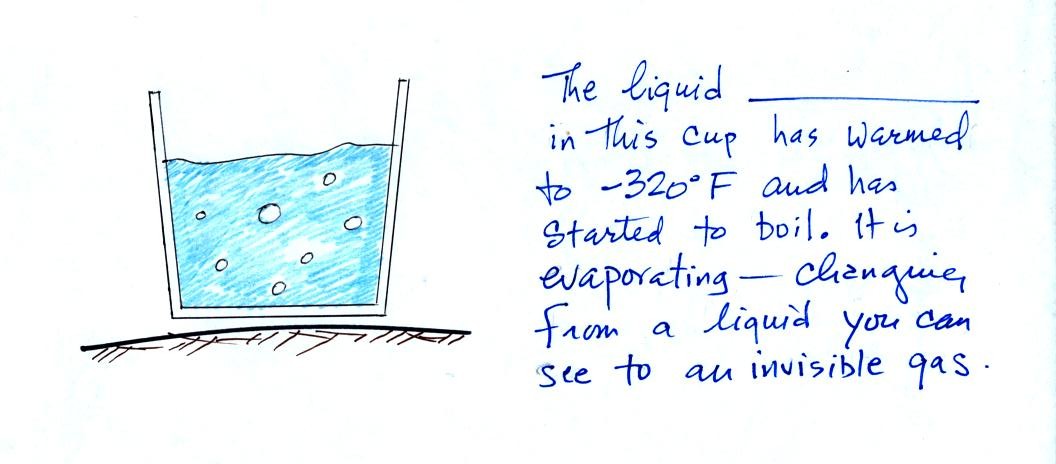
We could use the cup of liquid
nitrogen to show this.
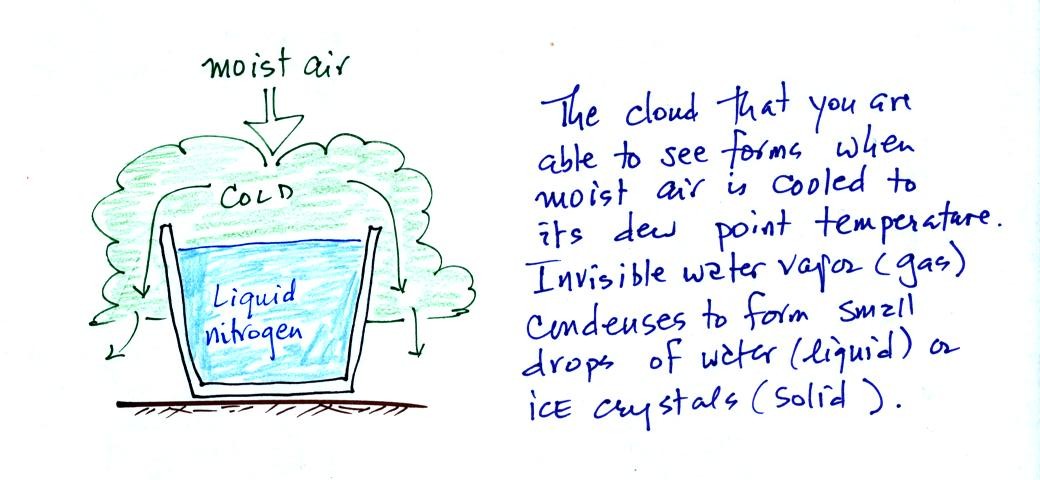
The cloud came from moisture in the
air. The cloud was not made of nitrogen gas (which is
invisible). Note also that a certain amount of "artistic" license
was used in the figure above; liquid nitrogen is not blue and water
clouds are not green.
This is where we finished up in class on Friday. I may add a
little bit more information over the weekend. If I do, I'll
review it quickly at the start of class next Wednesday.