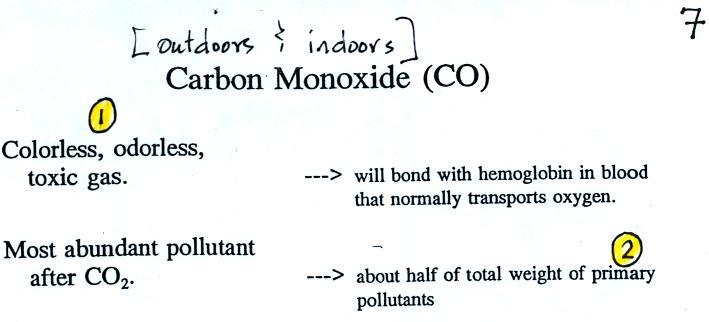
We will mostly be talking about
carbon
monoxide found outdoors, where it would rarely reach fatal
concentrations. Indoors is a serious hazard indoors also where it
can (and does) build up to deadly concentrations. (
several people were almost killed in Tucson last December)
Carbon monoxide is insidious, you can't smell it or see it and it can kill you (Point 1). Once inhaled, carbon monoxide molecules bond strongly to the hemoglobin molecules in blood and interfere with the transport of oxygen throughout your body.
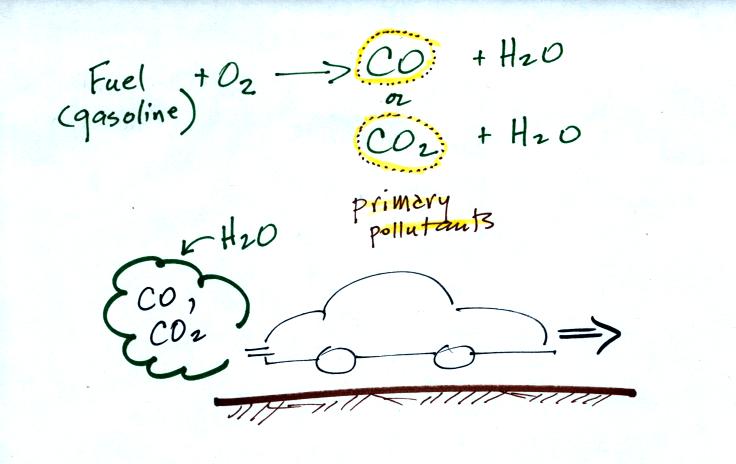
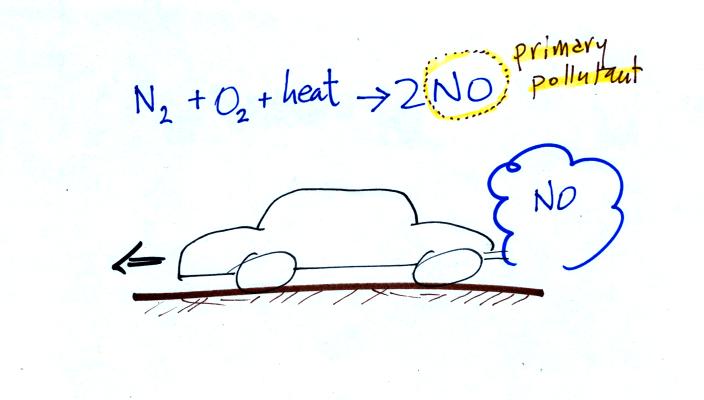
CO is a primary pollutant (Point 2 above). That means it goes directly from a source into the air, CO is emitted directly from an automobile tailpipe into the atmosphere for example. The difference between primary and secondary pollutants is probably explained best in a series of pictures.
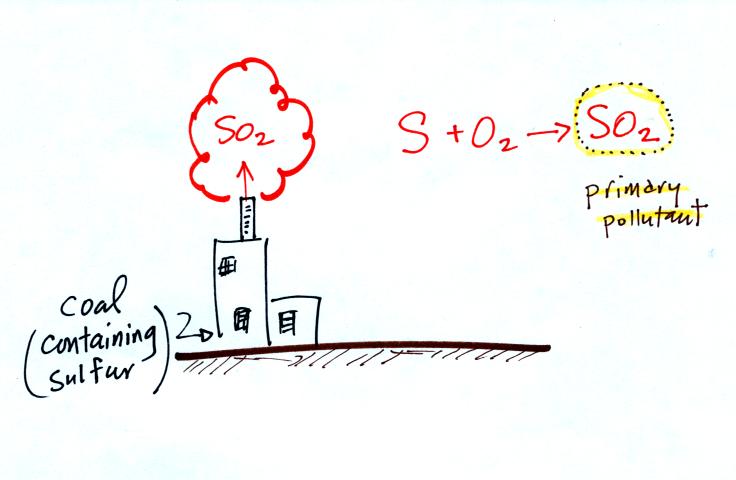
Nitric oxide, NO, and sulfur dioxide, SO2, are also primary pollutants. They all go directly from a source (automobile tailpipe or factory chimney) into the atmosphere. Ozone is a secondary pollutant (and here we are referring to tropospheric ozone, not stratospheric ozone). It doesn't come directly from an automobile tailpipe. It shows up in the atmosphere only after a primary pollutant has undergone a series of reactions. This is where we left off in class on Wednesday.
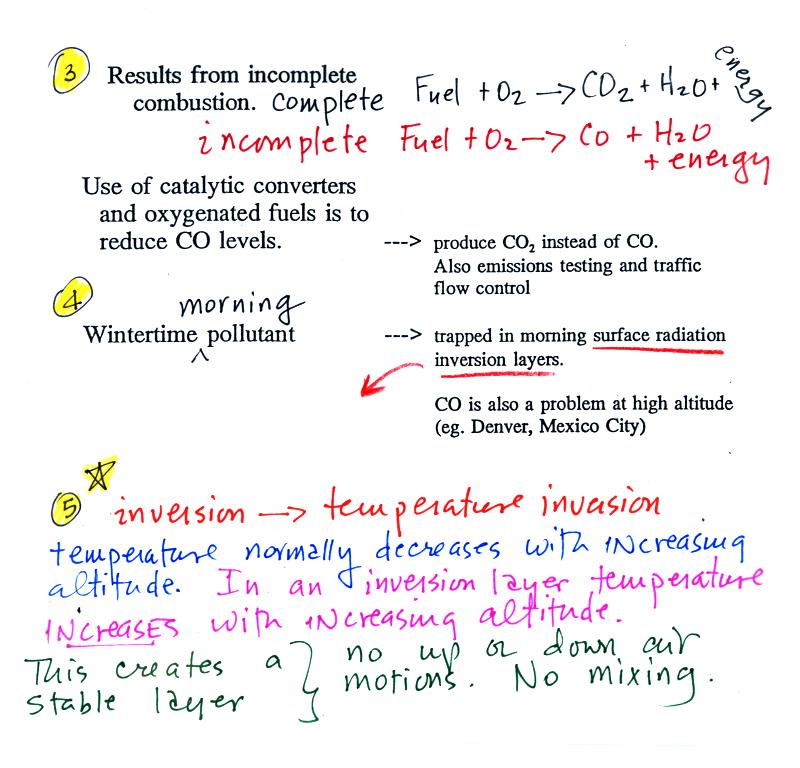
Point 3
explains that CO is produced by incomplete
combustion of fossil
fuel (insufficient oxygen). Complete combustion would produce
carbon dioxide,
CO2. Cars and trucks produce much of the CO in
the
atmosphere in Tucson.
Vehicles must now be fitted with a catalytic converter that will change CO into CO2 (and also NO into N2 and O2 and hydrocarbons into H2O and CO2). In Pima County vehicles must also pass an emissions test every year and special formulations of gasoline (oxygenated fuels) are used during the winter months to try to reduce CO emissions.
In the atmosphere CO concentrations peak on winter mornings (Point 4). The reason for this is surface radiation inversion layers. They are most likely to form on cold winter mornings.
In an inversion layer (Point 5) air temperature actually increases with increasing altitude which is just the opposite of what we are used to. This produces stable atmospheric conditions which means there is little up or down air motion.
There is very little vertical mixing in a stable air layer.
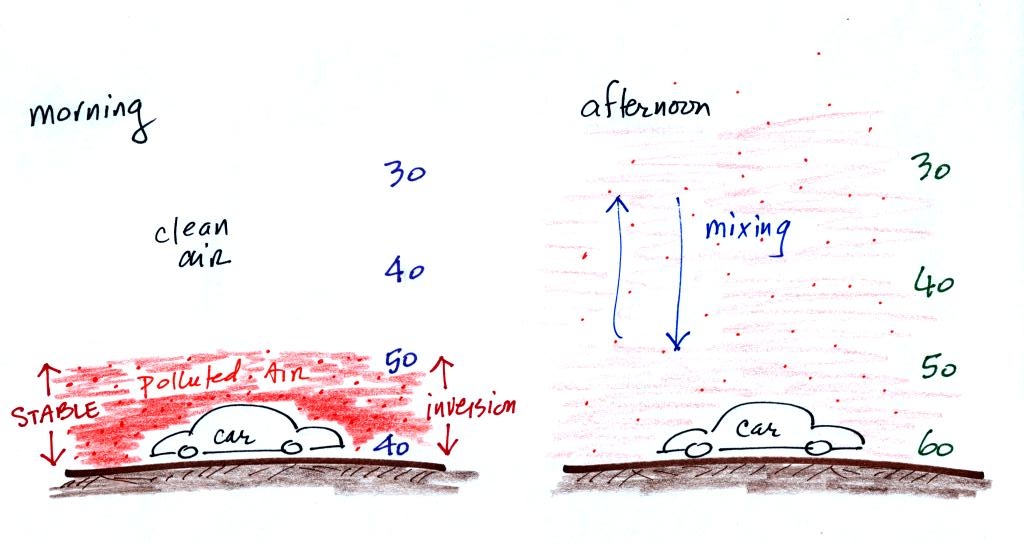
When CO is emitted into the thin stable layer (left figure above), the CO remains in the layer and doesn't mix with cleaner air above. CO concentrations build.
In the afternoon, the ground warms, and the atmosphere becomes more unstable. CO emitted into air at the surface mixes with cleaner air above. The CO concentrations are effectively diluted.
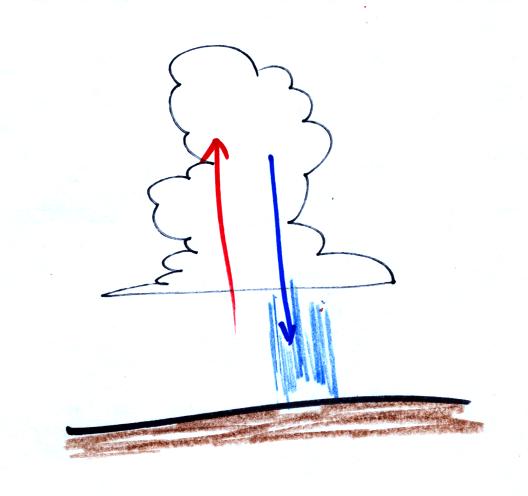
Thunderstorms
contain strong up
(updraft) and down (downdraft) air motions. Thunderstorms are a
sure indication of unstable
atmospheric conditions. When the
downdraft winds hit the ground they spread out horizontally.
These surface winds can sometimes reach 100 MPH, stronger than many
tornadoes.
The concentrations of several of the main pollutants are monitored in large cities in the US and around the world. Six pollutants are listed below (p. 8 in the photocopied ClassNotes). In Tucson, carbon monoxide, ozone, and particulate matter are of primary concern and daily measurements are reported in the city newspaper. The Air Quality Index value is reported instead of the actual concentration. The AQI is the ratio of the measured to accepted concentrations multiplied by 100%. Air becomes unhealthy when the AQI value exceeds 100%.

The atmospheric concentration of lead has decreased significantly since the introduction of unleaded gasoline. PM stands for particulate matter. These small particles are invisible, remain suspended in the air, and may be made of harmful materials. We'll talk about them in a little more detail next week.
For carbon monoxide, concentrations up to 35 ppm (parts per million) for a 1 hour period and 9 ppm for an 8 hour period are allowed.
Here are a couple of example calculations that weren't done in class:
If the observed CO concentration were 4.5 ppm averaged over an 8 hour period the AQI would be
What would the measured CO 8 hr average concentration be for an AQI value of 33%?
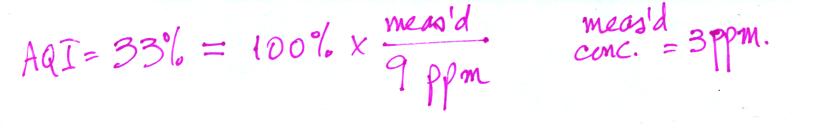
Current Air Quality Index values for Tucson are available online.
Carbon monoxide is a serious hazard indoors where is can build to much higher levels than would ever be found outdoors. I mentioned an incident at Virginia Tech (that occurred near the beginning of the school year in 2007). Carbon monoxide from a malfunctioning hot water heater sickened 23 Virginia Tech students in an apartment complex. The CO concentration is thought to have reached 500 ppm. You can get an idea of what kinds of health effects concentrations this high could cause from the figure. on p. 9 in the photocopied ClassNotes.
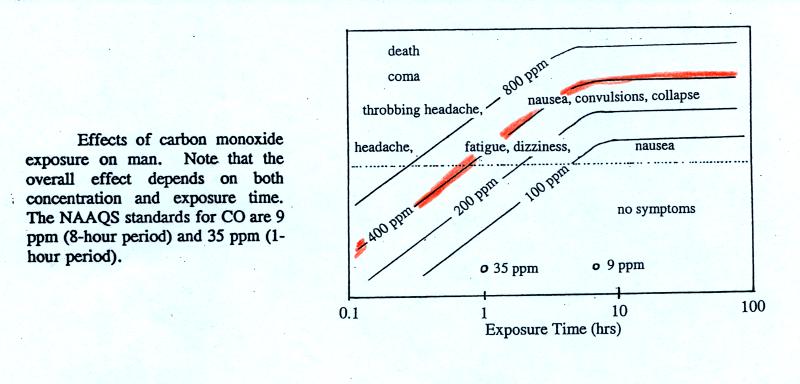
The 400
ppm line in the ClassNotes approaches the level where CO would cause
coma and
death. At Virginia Tech several students were found unconscious
and one or two had stopped breathing but they were revived.
Carbon monoxide alarms are relatively inexpensive (~$50) and readily available at most hardware stores. They will monitor CO concentrations indoors and warn you when concentrations reach hazardous levels. Indoors CO is produced by gas furnaces and water heaters that are either operating improperly or aren't being adequately vented to the outdoors. A few hundred people are killed indoors by carbon monoxide every year in the United States. You can learn more about carbon monoxide hazards and risk prevention at the Consumer Product Safety Commission web page.
You are able to see a lot of things in the atmosphere (clouds, fog, haze, even the blue sky) because of scattering of light. I'm going to try to make a cloud of smog in class next week. The individual droplets making up the smog cloud too small to be seen by the naked eye. But you will be able to see that they're there because the droplets scatter light. So we took some time for a demonstration that tried to show you exactly what light scattering is.
In the first part of the demonstration a narrow beam of intense
red
laser light was shined from one side of the classroom to the
other.
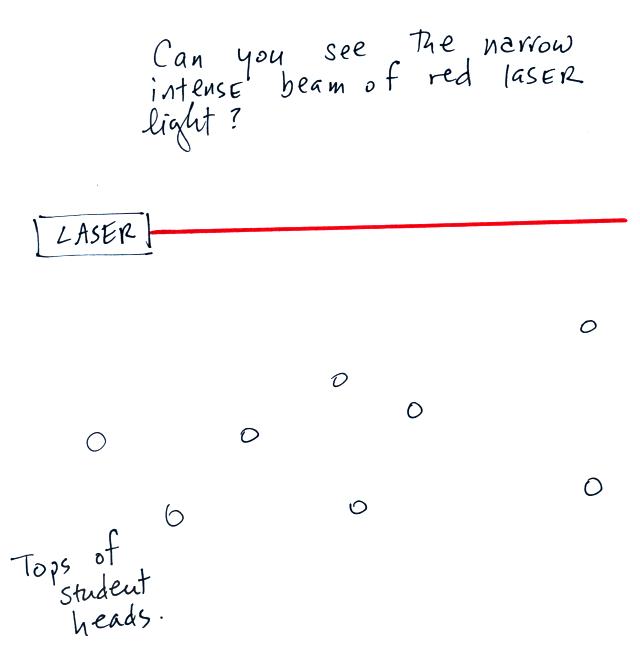
I asked the students in the room whether they could see the beam.
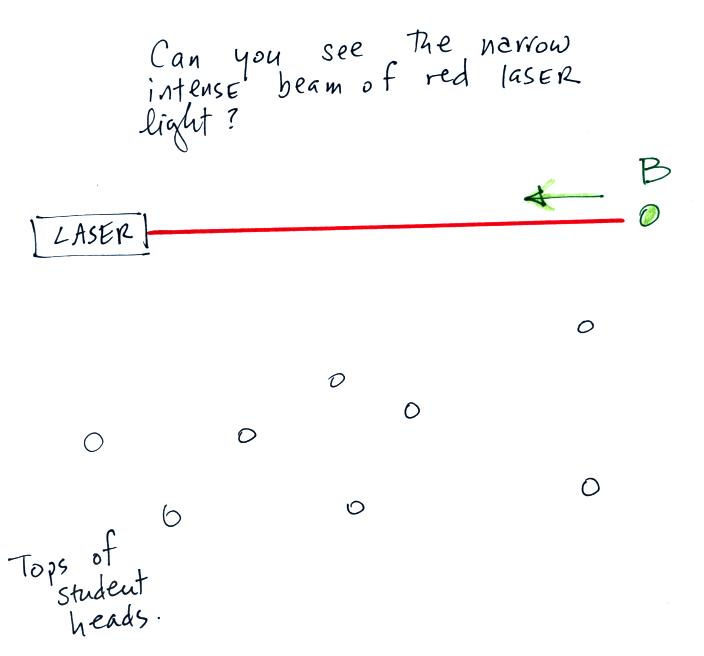
They told me they could not. The students couldn't see the laser beam because the light rays weren't pointing straight at them. The instructor would have been able to see the beam if he had stood at Point B in the figure above and looked back along the beam of light toward the laser (that wouldn't have been a smart thing to do because the beam is strong enough to damage his eyes).
Students were able to see a bright red spot where the laser beam struck the wall.
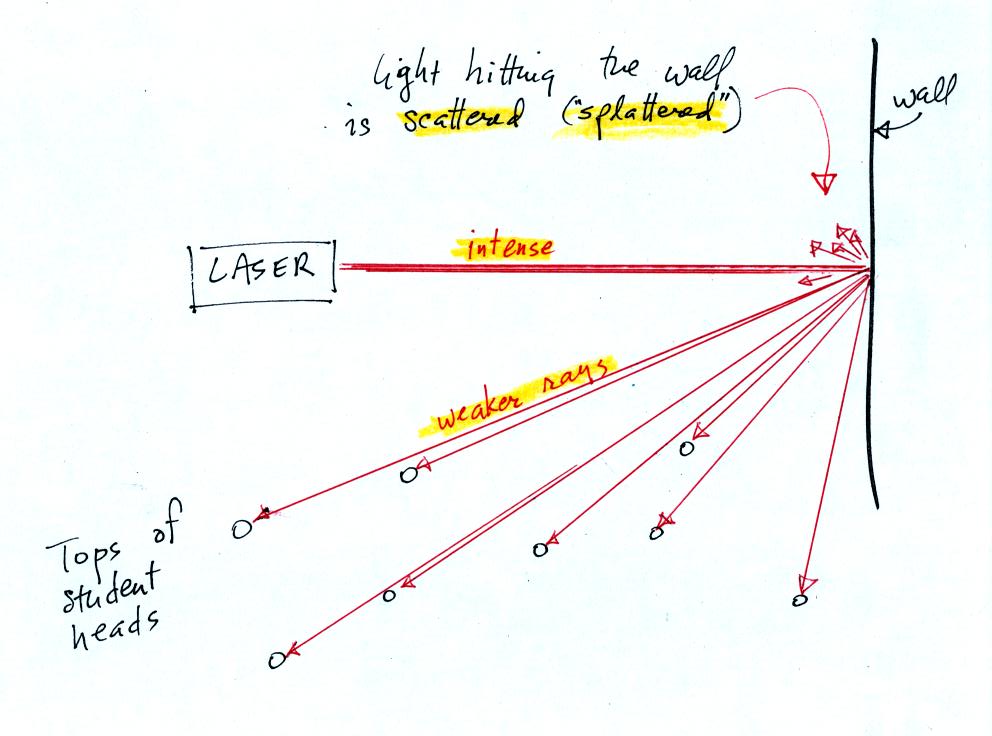
This is because when the intense beam of
laser light
hits the wall it
is scattered (splattered is a
more descriptive term). Weaker rays
of light are sent out in all directions. There is a ray of light
sent in the direction of every student in the class. They see the
light because they are looking back in the direction the ray came
from. It is safe to look at this light because the original
intense beam is split up into many much weaker beams.
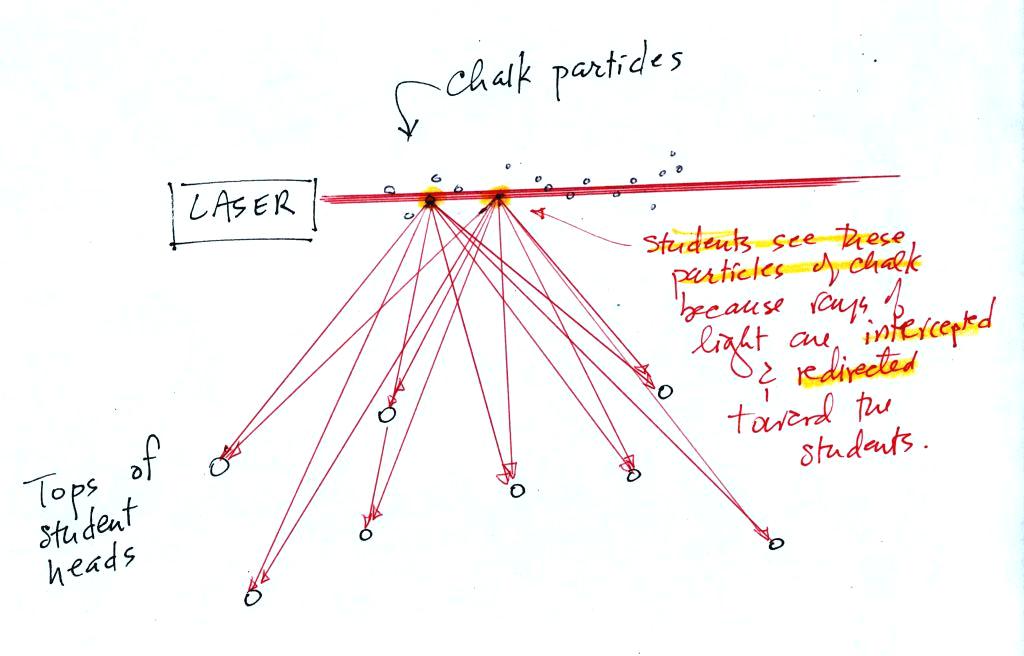
Next we clapped some erasers together so that some small particles of chalk dust fell into the laser beam.
Carbon monoxide is insidious, you can't smell it or see it and it can kill you (Point 1). Once inhaled, carbon monoxide molecules bond strongly to the hemoglobin molecules in blood and interfere with the transport of oxygen throughout your body.
CO is a primary pollutant (Point 2 above). That means it goes directly from a source into the air, CO is emitted directly from an automobile tailpipe into the atmosphere for example. The difference between primary and secondary pollutants is probably explained best in a series of pictures.
 |
 |
 |
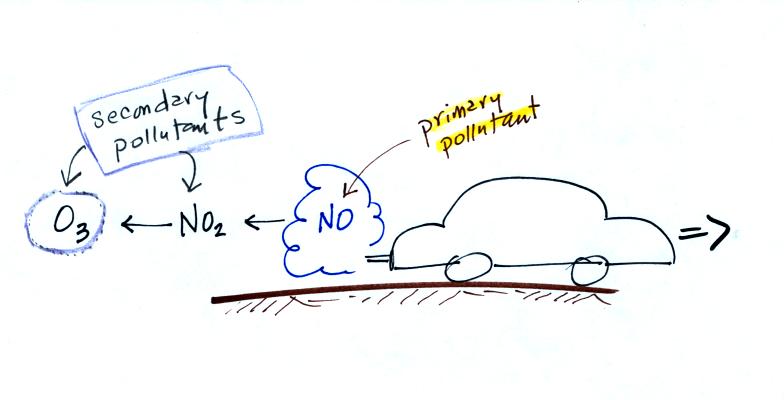 |
Nitric oxide, NO, and sulfur dioxide, SO2, are also primary pollutants. They all go directly from a source (automobile tailpipe or factory chimney) into the atmosphere. Ozone is a secondary pollutant (and here we are referring to tropospheric ozone, not stratospheric ozone). It doesn't come directly from an automobile tailpipe. It shows up in the atmosphere only after a primary pollutant has undergone a series of reactions. This is where we left off in class on Wednesday.

Vehicles must now be fitted with a catalytic converter that will change CO into CO2 (and also NO into N2 and O2 and hydrocarbons into H2O and CO2). In Pima County vehicles must also pass an emissions test every year and special formulations of gasoline (oxygenated fuels) are used during the winter months to try to reduce CO emissions.
In the atmosphere CO concentrations peak on winter mornings (Point 4). The reason for this is surface radiation inversion layers. They are most likely to form on cold winter mornings.
In an inversion layer (Point 5) air temperature actually increases with increasing altitude which is just the opposite of what we are used to. This produces stable atmospheric conditions which means there is little up or down air motion.
There is very little vertical mixing in a stable air layer.

When CO is emitted into the thin stable layer (left figure above), the CO remains in the layer and doesn't mix with cleaner air above. CO concentrations build.
In the afternoon, the ground warms, and the atmosphere becomes more unstable. CO emitted into air at the surface mixes with cleaner air above. The CO concentrations are effectively diluted.

The concentrations of several of the main pollutants are monitored in large cities in the US and around the world. Six pollutants are listed below (p. 8 in the photocopied ClassNotes). In Tucson, carbon monoxide, ozone, and particulate matter are of primary concern and daily measurements are reported in the city newspaper. The Air Quality Index value is reported instead of the actual concentration. The AQI is the ratio of the measured to accepted concentrations multiplied by 100%. Air becomes unhealthy when the AQI value exceeds 100%.

The atmospheric concentration of lead has decreased significantly since the introduction of unleaded gasoline. PM stands for particulate matter. These small particles are invisible, remain suspended in the air, and may be made of harmful materials. We'll talk about them in a little more detail next week.
For carbon monoxide, concentrations up to 35 ppm (parts per million) for a 1 hour period and 9 ppm for an 8 hour period are allowed.
Here are a couple of example calculations that weren't done in class:
If the observed CO concentration were 4.5 ppm averaged over an 8 hour period the AQI would be
AQI = 100% x (4.5ppm / 9ppm) = 50%
and the air quality would be considered good.What would the measured CO 8 hr average concentration be for an AQI value of 33%?
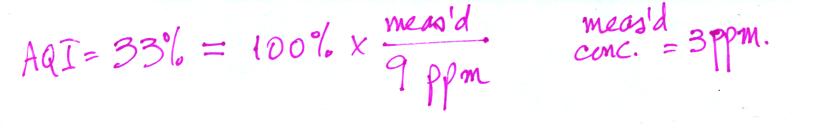
Current Air Quality Index values for Tucson are available online.
Carbon monoxide is a serious hazard indoors where is can build to much higher levels than would ever be found outdoors. I mentioned an incident at Virginia Tech (that occurred near the beginning of the school year in 2007). Carbon monoxide from a malfunctioning hot water heater sickened 23 Virginia Tech students in an apartment complex. The CO concentration is thought to have reached 500 ppm. You can get an idea of what kinds of health effects concentrations this high could cause from the figure. on p. 9 in the photocopied ClassNotes.

Carbon monoxide alarms are relatively inexpensive (~$50) and readily available at most hardware stores. They will monitor CO concentrations indoors and warn you when concentrations reach hazardous levels. Indoors CO is produced by gas furnaces and water heaters that are either operating improperly or aren't being adequately vented to the outdoors. A few hundred people are killed indoors by carbon monoxide every year in the United States. You can learn more about carbon monoxide hazards and risk prevention at the Consumer Product Safety Commission web page.
You are able to see a lot of things in the atmosphere (clouds, fog, haze, even the blue sky) because of scattering of light. I'm going to try to make a cloud of smog in class next week. The individual droplets making up the smog cloud too small to be seen by the naked eye. But you will be able to see that they're there because the droplets scatter light. So we took some time for a demonstration that tried to show you exactly what light scattering is.
other.

I asked the students in the room whether they could see the beam.

They told me they could not. The students couldn't see the laser beam because the light rays weren't pointing straight at them. The instructor would have been able to see the beam if he had stood at Point B in the figure above and looked back along the beam of light toward the laser (that wouldn't have been a smart thing to do because the beam is strong enough to damage his eyes).
Students were able to see a bright red spot where the laser beam struck the wall.

Next we clapped some erasers together so that some small particles of chalk dust fell into the laser beam.

Now instead
of a single spot on the wall, students
saws lots of
points of light coming from different positions in a straight line
along the laser
beam. Each of these points of light was a particle of chalk, and
each piece of chalk dust was intercepting laser light and sending light
out in all directions. Each student saw a ray of light coming
from
each of the chalk particles.
We use chalk because it is white, it will scatter rather than absorb visible light. What would you have seen if black particles of soot had been dropped into the laser beam?
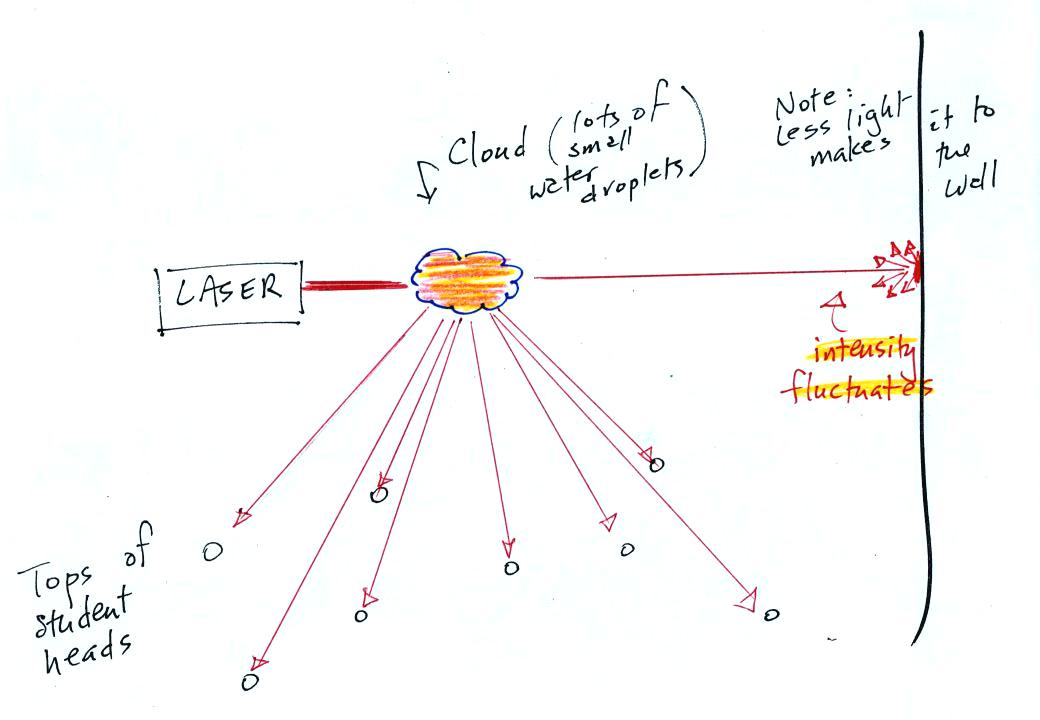
In the last part of the demonstration we made a cloud by pouring some liquid nitrogen into a cup of water. The cloud droplets are much smaller than the chalk particles but are much more numerous. They made very good scatterers.
We use chalk because it is white, it will scatter rather than absorb visible light. What would you have seen if black particles of soot had been dropped into the laser beam?
In the last part of the demonstration we made a cloud by pouring some liquid nitrogen into a cup of water. The cloud droplets are much smaller than the chalk particles but are much more numerous. They made very good scatterers.

The laser
light really lit up and
turned the small patches of
cloud
red. The cloud did a very good job of scattering laser light. So
much light was scattered
that the spot on the wall fluctuated in intensity (the spot dimmed when
lots of
light was being scattered, and brightened when not as much light was
scattered).
Here's a comment that wasn't mentioned in class Air molecules are able to scatter light too, just like cloud droplets. Air molecules are much smaller than cloud droplets and don't scatter much light. That's why you weren't able to see light being scattered by air before we put chalk particles or cloud droplets into the beam. Outdoors you are able to see sunlight (much more intense than the laser beam used in the class demonstration) scattered by air molecules. Sunlight is white and is made up of violet, blue, green, yellow, orange, and red light. Air molecules have an unusual property: they scatter the shorter wavelengths (violet, blue, green) much more readily than the longer wavelength colors in sunlight (yellow, orange, and red). When you look away from the sun and look at the sky, the blue color that you see are the shorter wavelengths in sunlight that are being scattered by air molecules.
We'll come back to the topic of light scattering next week. when we cover particulate matter and its effect on visibility.
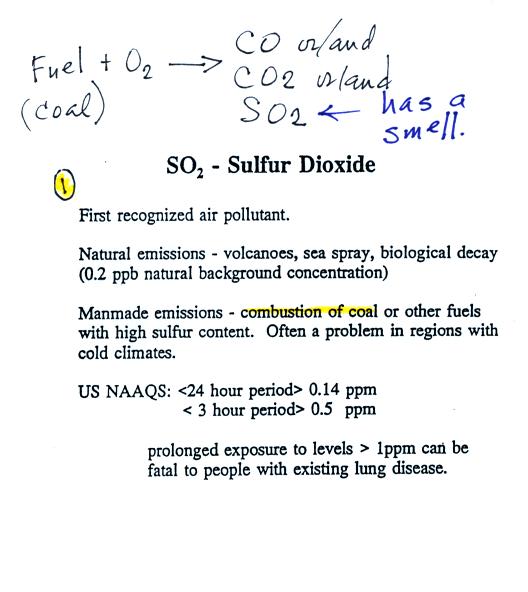
We had enough time to just get started on the 2nd pollutant we will cover - sulfur dioxide. Here's some basic information from the left hand of p. 11 in the photocopied ClassNotes.

The
Great
London smog is still one of the two or three deadliest air pollution
events in
history.
Because of a subsidence inversion the atmosphere was stable
SO2 emitted into air at ground level couldn't mix with cleaner air above.
The SO2 concentration was able to build to dangerous levels.
4000 people died during this 4 or 5 day period.
As many as 8000 additional people died in the following weeks and months.
Some of the photographs below come from articles published in 2002 on the 50th anniversary of the event.
The sulfur dioxide didn't kill people directly.
The SO2 aggravated an existing condition of some kind and hastened their death.
The SO2 probably also made people susceptible to bacterial infections such as pneumonia.
This link discusses the event and its health effects in more detail.
Note:
London type smog which contains sulfur dioxide and is most common during the winter is very different from photochemical or Los Angeles type smog, something we will be learning about next week. Los Angeles type smog contains ozone and is most common in the summer.
Some other air pollution disasters also involved high SO2 concentrations.
One of the deadliest events in the US occurred in 1948 in Donora, Pennsylvania.

"This eerie photograph was taken at noon on Oct. 29, 1948 in Donora, PA as deadly smog enveloped the town. 20 people were asphyxiated and more than 7,000 became seriously ill during this horrible event."
from: http://oceanservice.noaa.gov/education/kits/pollution/02history.html

from: http://www.eoearth.org/article/Donora,_Pennsylvania
"When Smoke Ran Like Water," a book about air pollution is among the books that you can check out, read, and report on to fulfill part of the writing requirements in this class (instead of doing an experiment report). The author, Devra Davis, lived in Donora Pennsylvania at the time of the 1948 air pollution episode.
Here's a figure I didn't cover in class. This is an example of a habit I have of "beating some concepts to death." The rather busy picture below illustrates how small changes in how air temperature changes with increasing altitude can determine whether the atmosphere will be stable or unstable. Just for the purposes of illustration imagine riding a bicycle north from Swan and River Rd up the hill to Swan and Sunrise (fhe figure shows an elevation change of 1000 ft, it is actually quite a bit less than that).

At far left the air temperature goes from 47o F to 41o F, a drop of 6o F. This is a fairly rapid rate of decrease with increasing altitude and would make the atmosphere absolutely unstable. The atmosphere wouldn't remain this way. Air at the ground would rise, air higher up would sink, and the temperature profile would change (the rate of decrease with increasing altitude would lessen). In some ways it would be like trying to pour vinegar on top of oil in a glass. The lower density oil would rise because it would "want" to float on top of the higher density vinegar.
The next picture shows air temperature decreasing a little more slowly with increasing altitude. This small change makes the atmosphere conditionally unstable (we won't go into what the conditions might be). The atmosphere is often in this state.
The atmosphere cools only 2o F in 1000 feet in the next picture. This creates an absolutely stable atmosphere. Air at the ground will remain at the ground and won't rise and mix with air higher up. Compare this with the glass containing vinegar and a layer of oil on top. The two layers won't mix.
Air temperature in the last figure actually increases with increasing altitude. This is a temperature inversion and is very common on winter mornings. The atmosphere is extremely stable under these conditions.
Temperature inversions are something you can check out for yourself later this semester. Head north on Swan Rd. on your bicycle early some winter morning. You will pass through some pretty cold air as you cross the Rillito River. By the time you get to Sunrise, the air can be 10 to 15 degrees warmer and will seem balmy compared to the cold air at the bottom of the hill. If you're up for a real hill-climbing challenge continue north on Swan past Skyline. You'll find a short but very steep section of road at the far north end of Swan.
Here's a comment that wasn't mentioned in class Air molecules are able to scatter light too, just like cloud droplets. Air molecules are much smaller than cloud droplets and don't scatter much light. That's why you weren't able to see light being scattered by air before we put chalk particles or cloud droplets into the beam. Outdoors you are able to see sunlight (much more intense than the laser beam used in the class demonstration) scattered by air molecules. Sunlight is white and is made up of violet, blue, green, yellow, orange, and red light. Air molecules have an unusual property: they scatter the shorter wavelengths (violet, blue, green) much more readily than the longer wavelength colors in sunlight (yellow, orange, and red). When you look away from the sun and look at the sky, the blue color that you see are the shorter wavelengths in sunlight that are being scattered by air molecules.
We'll come back to the topic of light scattering next week. when we cover particulate matter and its effect on visibility.
We had enough time to just get started on the 2nd pollutant we will cover - sulfur dioxide. Here's some basic information from the left hand of p. 11 in the photocopied ClassNotes.

Sulfur dioxide is produced by the
combustion of sulfur
containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless. That is why sulfur
dioxide was the first pollutant people became aware of.
Volcanoes are a natural source of sulfur dioxide.
Volcanoes are a natural source of sulfur dioxide.

Because of a subsidence inversion the atmosphere was stable
SO2 emitted into air at ground level couldn't mix with cleaner air above.
The SO2 concentration was able to build to dangerous levels.
4000 people died during this 4 or 5 day period.
As many as 8000 additional people died in the following weeks and months.
Some of the photographs below come from articles published in 2002 on the 50th anniversary of the event.
The sulfur dioxide didn't kill people directly.
The SO2 aggravated an existing condition of some kind and hastened their death.
The SO2 probably also made people susceptible to bacterial infections such as pneumonia.
This link discusses the event and its health effects in more detail.
Note:
London type smog which contains sulfur dioxide and is most common during the winter is very different from photochemical or Los Angeles type smog, something we will be learning about next week. Los Angeles type smog contains ozone and is most common in the summer.
Some other air pollution disasters also involved high SO2 concentrations.
One of the deadliest events in the US occurred in 1948 in Donora, Pennsylvania.

"This eerie photograph was taken at noon on Oct. 29, 1948 in Donora, PA as deadly smog enveloped the town. 20 people were asphyxiated and more than 7,000 became seriously ill during this horrible event."
from: http://oceanservice.noaa.gov/education/kits/pollution/02history.html

"When Smoke Ran Like Water," a book about air pollution is among the books that you can check out, read, and report on to fulfill part of the writing requirements in this class (instead of doing an experiment report). The author, Devra Davis, lived in Donora Pennsylvania at the time of the 1948 air pollution episode.
Here's a figure I didn't cover in class. This is an example of a habit I have of "beating some concepts to death." The rather busy picture below illustrates how small changes in how air temperature changes with increasing altitude can determine whether the atmosphere will be stable or unstable. Just for the purposes of illustration imagine riding a bicycle north from Swan and River Rd up the hill to Swan and Sunrise (fhe figure shows an elevation change of 1000 ft, it is actually quite a bit less than that).

At far left the air temperature goes from 47o F to 41o F, a drop of 6o F. This is a fairly rapid rate of decrease with increasing altitude and would make the atmosphere absolutely unstable. The atmosphere wouldn't remain this way. Air at the ground would rise, air higher up would sink, and the temperature profile would change (the rate of decrease with increasing altitude would lessen). In some ways it would be like trying to pour vinegar on top of oil in a glass. The lower density oil would rise because it would "want" to float on top of the higher density vinegar.
The next picture shows air temperature decreasing a little more slowly with increasing altitude. This small change makes the atmosphere conditionally unstable (we won't go into what the conditions might be). The atmosphere is often in this state.
The atmosphere cools only 2o F in 1000 feet in the next picture. This creates an absolutely stable atmosphere. Air at the ground will remain at the ground and won't rise and mix with air higher up. Compare this with the glass containing vinegar and a layer of oil on top. The two layers won't mix.
Air temperature in the last figure actually increases with increasing altitude. This is a temperature inversion and is very common on winter mornings. The atmosphere is extremely stable under these conditions.
Temperature inversions are something you can check out for yourself later this semester. Head north on Swan Rd. on your bicycle early some winter morning. You will pass through some pretty cold air as you cross the Rillito River. By the time you get to Sunrise, the air can be 10 to 15 degrees warmer and will seem balmy compared to the cold air at the bottom of the hill. If you're up for a real hill-climbing challenge continue north on Swan past Skyline. You'll find a short but very steep section of road at the far north end of Swan.



