Once the cloud had formed we shined
the laser beam through the flask. Laser light wasn't visible to
the left or the right of the flask, only in the flask where smog
droplets were present and scattering laser light. The smog
droplets (and they may well be solid particles, I don't know) are very
small and even the weakest air current is able to keep them suspended.
As long as
we're talking about ozone it seemed reasonable to learn a little bit
more about stratospheric ozone. Stratospheric ozone (the ozone
layer)
absorbs dangerous high-energy ultraviolet light.
This topic is covered on pps. 17-19 in the photocopied ClassNotes.
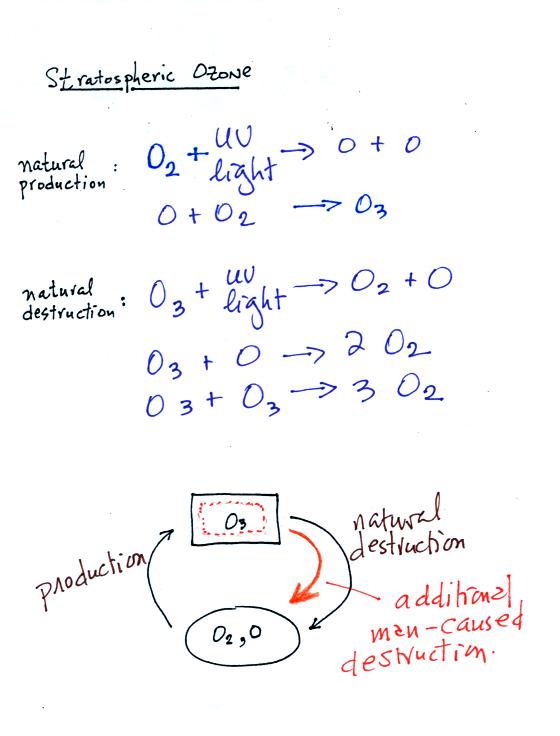
The
top
two
equations
show
how ozone is produced naturally in the stratosphere.
Ultraviolet (UV) light splits an O2 molecule into two O atoms
(photodissociation).
Each of the O atoms can react with O2 to make O3 (ozone).
Ozone is destroyed when it absorbs UV light and is split into O
and O2
(the two pieces move away from each other and don't recombine and
remake ozone). O3 is also destroyed when it reacts with an oxygen
atom (thereby removing one of the "raw ingredients" used to make
ozone). Two molecules of ozone can also react to make 3 molecules
of O2.
The bottom part of the figure attempts to show that the ozone
concentration in the
stratosphere will change until the natural rates of
production and
destruction
balance each other (analogous to your bank account not changing when
the amount of money deposition and withdrawn are equal). If an
additional man-caused destruction process is added (orange) that will
lower the ozone layer concentration (if someone else starts spending
some of your money, you balance will decrease).
Knowing that you need O2 and UV light to make ozone,
you can
begin
to understand why the ozone layer is found in the middle of the
atmosphere.
Three different types of UV light were mentioned in class: UVA, UVB,
and UVC. UVA is a relatively long wavelength (0.315 to 0.4
micrometers), low energy form of UV light; it is the light emitted by a
"black light".
UVB
has
shorter wavelength (0.28 to 0.315 micrometers). UVC has
even shorter wavelength (0.1 to 0.28 micrometers) and is the most
dangerous of the three types of UV light. Germicidal
bulbs emit UVC light and are used to sterilize and purify air,
food, and water. All of the UVC in sunlight is absorbed by the
upper atmosphere.
There is some question
about whether
tanning booths, which emit mostly UVA, are safe. You can find
information online about
this
question. Here is an
example from the US Food and Drug Administration.

The first set of reactions above involve nitric oxide, NO. First,
NO reacts with O3 to form NO2 and O2
(ordinary molecular oxygen).
Then notice the NO2 reacts with an oxygen atom (which might
otherwise
react with O2 to make O3) to form NO again and O2.
The
NO
is
available
again
to
react
with
and
destroy
another
ozone
molecule.
At one time many countries were considering building fleets of
supersonic aircraft that would fly into the stratosphere. The
plans were scrapped partly due to concern that the NO emissions from
these aircraft would damage the ozone layer.
The main threat now comes from chlorofluorocarbons (CFCs). CFCs
were
at
one
time
thought
to
be
an
ideal
industrial
chemical
and had a variety of uses.
CFCs are unreactive, non toxic, and stable. Once they get into
the atmosphere they remain there a long time, as much as 100 years.
The
reactions
involving
CFCs
are
shown
on
the
next
figure
(which was not
shown in class).
CFCs released at ground level [lower left corner in the figure
above]
remain in the atmosphere long enough that they can eventually make
their way up into the stratophere. UV light can then break
chlorine
atoms off the CFC molecule [a]. The resulting
"free chlorine" can react with and destroy ozone. This is shown
in (b) above. Note how the chlorine atoms reappears at the end of
the two step reaction. A single chlorine atom can destroy 100,000
ozone molecules.
There are ways of removing chlorine from the atmosphere. A
couple
of these so called "interference reactions" are shown in (c)
above. The reaction products, reservoir molecules
(because they store chlorine), might serve as
condensation nuclei for cloud droplets (the small water drops that
clouds are composed of) or might dissolve in the
water in clouds. In either event the chlorine containing chemical
is removed from the atmosphere by falling precipitation. Clouds
are probably the most effective way of cleaning the atmosphere.
Next we will be learning about some
of the physical properties of the atmosphere such as temperature, air
density, and air pressure. We'll also be interested in how they
change with altitude

Before we can learn about
atmospheric pressure in particular, we
need to review
the terms mass and weight. In some textbooks you'll find mass
defined as "amount of stuff" or "amount of a particular
material." Other books will define mass as
inertia or as resistance to change in motion (this comes from Newton's
2nd law of motion, we'll cover that later in the semester). The
next picture
illustrates both these definitions.

A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down than the
Volkswagen once it is
moving).
When
gravity
is
always
the
same,
three
objects
with
the
same
weight
would
also
have the same mass.
The
difference
between
mass
and
weight
is
clearer
(perhaps)
if
you
compare
the situation on the earth and on the moon.
On the earth a brick with a mass of about 2 kg weighs about 5
pounds. If you carried the brick to the moon it would have the
same mass. But gravity on the moon is weaker than on the
earth. Objects on the moon weigh less than on the earth.















