Mon., Mar. 26, 2012
click here to
download today's notes in a more printer friendly format
A couple of songs from Mariachi El Bronx ("Poverty's King"
and "Norteno Nights")
before
class
today.
The Experiment #3 reports were collected
today. It usually takes at least 1 week to grade them so you
should expect to get them back sometime next week.
We're getting to the point in this semester where you should already
have completed an experiment report or currently be working on an
experiment. Here's a list of
people that don't seem to have done
that yet. If you're on this list you should get in touch with me
right away.
The Controls of Temperature assignment was also collected together with
the Optional Assignment that was handed out in class last Friday.
Answers to the questions on the first assignment will appear online
soon. You'll find answers to the questions on the 2nd and more
challenging assignment at the end of today's notes.
In class last Friday we looked at the formation of dew, frozen dew, and
frost. Today we will be looking at what happens when air above
the ground is cooled to and below the dew point temperature (i.e. we'll
be looking at Pts. 2, 3, 4 and 5 in the figure below).
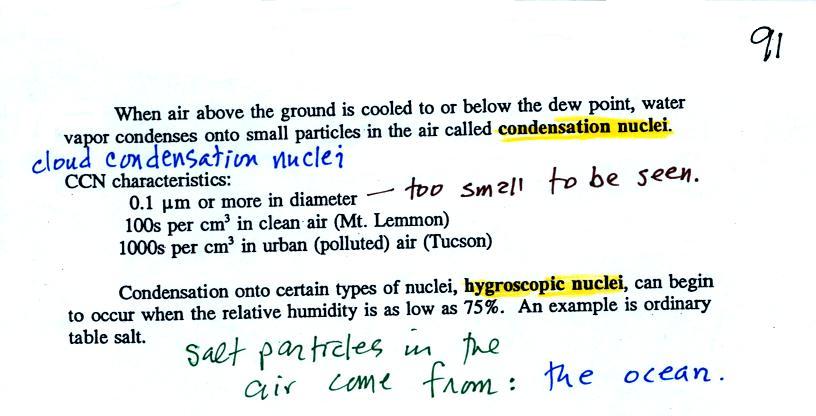
When the
relative humidity in air above the ground (and away from objects on the
ground) reaches 100%, water vapor will condense onto small particles
called condensation nuclei. It would be much harder for the water
vapor to just condense and form small droplets of pure water (you can
learn why that is so by reading the
top
of
p. 92 in the
photocopied class notes). There are always lots of CCN (cloud
condensation nuclei in the air) so this isn't an impediment to cloud
formation.
Water vapor will condense onto
certain kinds of condensation
nuclei
even when the relative humidity is below 100% (again you will find some
explanation of this on the bottom of p. 92).
These
are
called
hygroscopic
nuclei. Salt is an example; small particles of salt
mostly come from evaporating drops of ocean water.
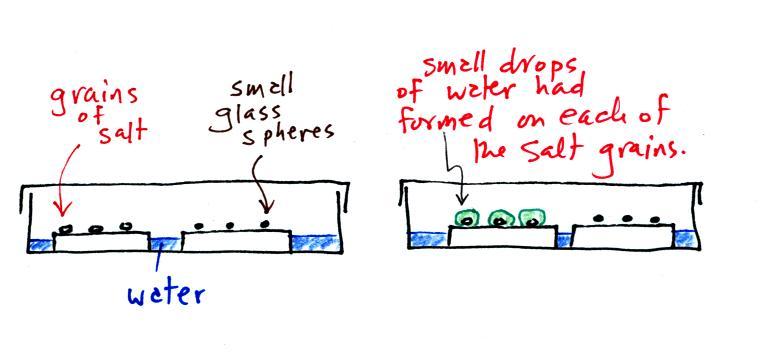
A short homemade video (my first actually) that showed how water
vapor would,
over time,
preferentially
condense onto small grains of salt rather than small spheres of
glass. The
figure
below
wasn't
shown
in
class.
The start of the video at left
showed the small grains
of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt but not the glass grains (shown above at
right).
In
humid parts of the US, water will condense onto the grains of
salt
in a salt shaker causing them to stick together. Grains of rice
apparently absorb moisture which keeps this from happening and also
break up lumps of salt once they start to form. Grains of rice
might also be used because they won't fall out of the holes in the salt
shaker together with the salt. You'll find this discussed in an interesting Wikipedia
article about salt.
The
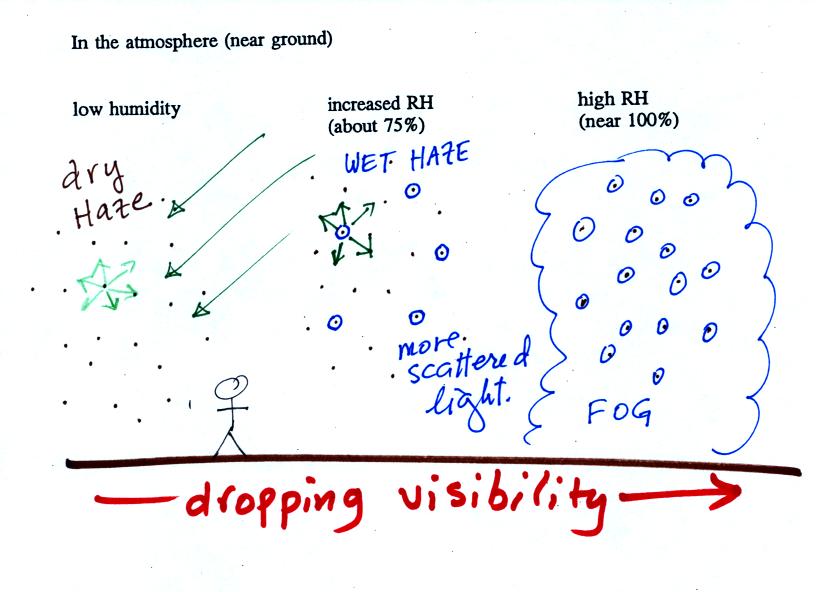
following figure is at the bottom of p. 91 in the ClassNotes.
This figure shows
how
cloud
condensation nuclei and increasing relative humidity can affect the
appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even
though
the condensation nuclei particles are too small to be seen with the
human eye you can tell they are there because they scatter
sunlight. When you look at the sky you see the deep blue color
caused by scattering of sunlight by air molecules mixed together with
some white
sunlight scattered by the condensation nuclei. This changes
the color of the sky from a deep blue to a bluish white
color. The more particles there are the whiter the sky
becomes. This is called "dry haze." Visibility under these
conditions might be a few tens of miles.
The middle picture shows what happens when you drive from the dry
southwestern part of the US into the humid
southeastern US or the Gulf Coast. One of the first things you
would notice is the
hazier
appearance of the air and a decrease in visibility. Because the
relative humidity is high,
water vapor begins to condense onto some of the condensation nuclei
particles (the hygroscopic nuclei) in the air and forms small water
droplets. The water droplets scatter more sunlight than just
small particles alone. The increase in the amount of scattered
light is what gives the air its hazier appearance. This is called "wet
haze." Visibility now might now only be a few miles.
Finally when the relative humidity increases to 100% fog
forms.
Fog can cause a severe drop in the visibility. The thickest fog
forms in dirty air that contains lots of condensation nuclei.
That is part of the reason the Great London Smog of 1952 was so
impressive. Visibility was at times just a few feet! We
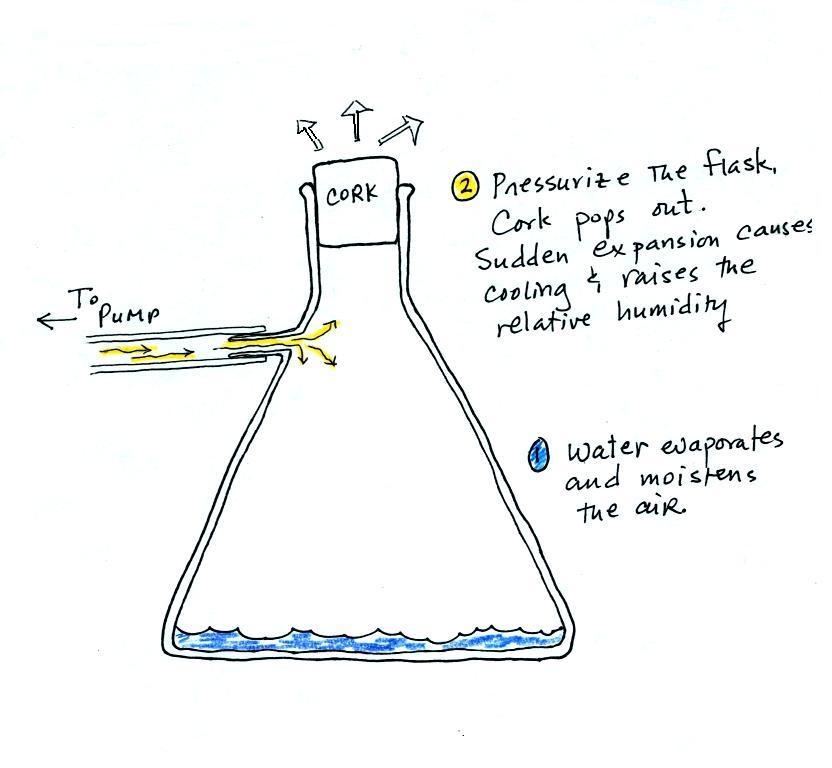
could see this effect in the cloud-in-a-bottle demonstration that was
performed next.
Cooling air, changing relative humidity, condensation
nuclei, and scattering of
light are all involved in this demonstration.
We used my backup flask in class. Normally I use use a
strong, thick-walled, 4 liter vacuum flask (designed to not implode
when all of the air is pumped out
of them, they aren't designed to not explode when pressurized).
There
was a little
water in the bottom of the flask to moisten the air in the flask.
Next we pressurized the air in the flask with a bicycle pump. At
some point the
pressure blows the cork out of the top of the flask.
The air in
the flask expands outward and cools. This sudden cooling
increases the
relative humidity of the moist air in the flask to 100% ( probably more
than 100% momentarily ) and water vapor condenses onto cloud
condensation nuclei in
the air. A very faint cloud became visible at this point.

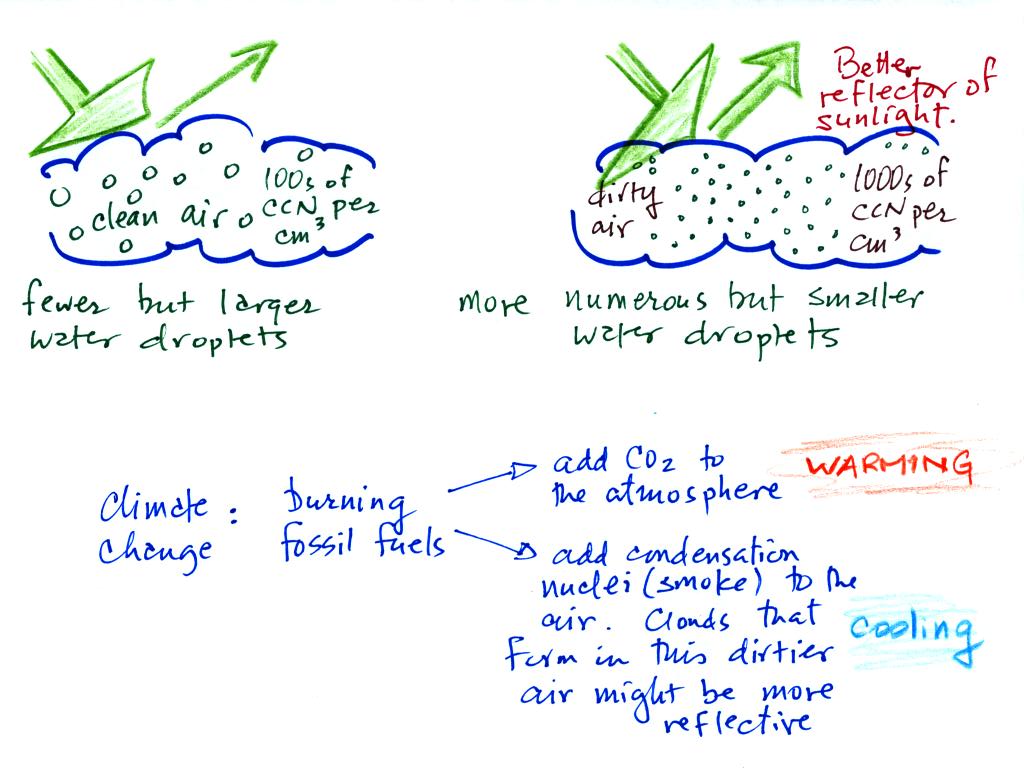
This effect has some implications for climate change.
A cloud that forms in dirty air is composed of a large
number of small droplets (right figure above). This cloud is more
reflective
than a cloud that forms in clean air, that is composed of a smaller
number of larger
droplets (left figure).
Combustion of fossil fuels adds carbon dioxide to the atmosphere.
There is concern that increasing carbon dioxide concentrations (and
other greenhouse gases) will
enhance the greenhouse effect and cause global warming.
Combustion also adds condensation nuclei to the atmosphere (just like
the burning match added smoke to the air in the flask). More
condensation nuclei might make it easier for clouds to form, might make
the clouds more reflective, and might cause cooling. There is
still quite a bit of uncertainty about how clouds might change and how
this
might affect climate. Remember that clouds are good absorbers of
IR radiation and also emit IR radiation.
Clouds are
one of
the best ways of cleaning the atmosphere

A cloud is composed of small water droplets (diameters of 10 or 20
micrometers) that form on particles ( diameters of perhaps 0.1 or 0.2
micrometers). The droplets "clump"
together to form a
raindrop (diameters of 1000 or 2000 micrometers which is 1 or 2
millimeters), and the raindrop carries the particles to the
ground.
A typical raindrop can contain 1 million cloud droplets so a single
raindrop
can remove a lot of particles from the air. You may have noticed
how clear the air seems the day after a rainstorm; distant mountains
are crystal clear and the sky has a deep blue color. Gaseous
pollutants can dissolve in the water droplets and be carried to
the ground by rainfall also. We'll be looking at the formation of
precipitation later this week.
And here, in a painful piece by piece kind of way, are answers to
the questions on the challenging assignment handed out in class last
Friday.

Here is, I think, the easiest part
of the 1st question. What will happen to the values of the mixing
ratio, r, the relative humidity, RH, and the dew point
temperature, Td, if you add moisture to or remove moisture
from a parcel of air?
The job of the mixing ratio is to tell you how much water vapor is
actually in the air. One of the jobs of the dew point is to do
the same thing. So both of these variables will increase when you
add moisture to the air and decrease when moisture is removed from the
air.
The value of the relative humidity depends on both the mixing ratio, r,
and
the
saturation mixing ratio rs. The saturation
mixing ratio depends on air temperature (warm air can potentially hold
more water vapor than colder air). But in this part of the
question temperature is remaining constant, so the value of rs
won't
change.
RH will change in the same way as r and
Td.

In this part of the problem we warm
the air but don't add or remove any moisture. Since moisture
isn't being added or removed the mixing ratio and the dew point
temperature will remain constant.
The relative humidity depends on r (which stays constant) and rs
(which
will
change because the air is warming). The value of rs
will
increase as the air is warmed. Since it is in the denominator of
the RH equation, the RH will decrease.

This is just the opposite
situation. We're cooling air but not adding or removing moisture
(as long as you don't cool the air below it's dew point
temperature). The values of r and T d will remain the same.
The RH will increase (eventually reaching 100% when you cool the air to
the dew point). RH decreases because cooling the air decreases
the saturation mixing ratio.
Finally we look at what happens when you cool the air below the
dew point temperature. In a previous lecture we saw that this is
one way of removing moisture from air. It's like wringing
moisture out of a sponge.

The RH reaches 100% when the air
has cooled to the dew point. As you cool air below Td the air's
capacity for water vapor, the saturation mixing ratio, continues to
decrease. The air finds itself with more water vapor than it can
hold. The excess condenses. The mixing ratio and the dew
point temperature will decreases as the air loses water vapor.
The RH will remain at 100%, the highest it can get.
There were two problems on the other side of the page.
Td and r have the same job, telling
you how much water vapor is actually in the air. The city with
the highest dew point temperature will also have the highest mixing
ratio and the highest amount of water vapor in the air.
Saturation mixing ratio depends on temperature. The city
with the warmest air will have the highest rs and could
potentially hold the most water vapor.
Finally the difference between Ta and Td gives you an idea of the
relative humidity. A small difference means high relative
humidity and vice versa (no difference between Ta and Td means RH =
100%.).
The figure below gives the answers to the last question.